��Ŀ����
(9��)ԭ�������������ӵ�A��B��C��D��E��F���ֳ���Ԫ���У�A��B��C��D�Ƕ����ڷǽ���Ԫ�أ�B��C��Dͬ���ڣ�E��F�ǵ������ڵĽ���Ԫ�أ�F���������ܲ����ȫ�������±�����Ҫ���ϼۼ�ԭ�Ӱ뾶���ݣ�
��1��B��C��D����Ԫ�ص�һ��������ֵ��С�����˳���� (��Ԫ�ط���)��
��2��B���⻯������ԭ�Ӳ�ȡ �ӻ����ռ乹���� �Σ��� ����(����ԡ��Ǽ��ԡ�)��
��3��F2����NH3 �γ������ӵĽṹʽΪ ����ij����ɫ��Һ�м��백ˮ���γ���ɫ�������������백ˮ���������ܽ�����ɫ����Һ���ɵõ��������������ӵ�����д�������ܽ�����ӷ���ʽ ��
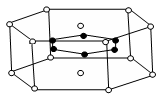
��4��A ��E����Ԫ���γɾ��徧������ͼ�е� (��١��ڡ��ۻ��)��
�� �� �� ��
��5����ͼ�������߷ֱ��ʾ��A�塢��A�塢��A�塢��A��Ԫ����̬�⻯��е�仯����ѡ��C���⻯�����ڵ����� ����n��m��x��y����
| | A | B | C | D | E | F |
| ��Ҫ���ϼ� | -1 | -3 +5 | -2 +6 | -1 +7 | +2 | +1 +2 |
| ԭ�Ӱ뾶 | 0.071 | 0.11 | 0.102 | 0.099 | 0.197 | 0.117 |
��2��B���⻯������ԭ�Ӳ�ȡ �ӻ����ռ乹���� �Σ��� ����(����ԡ��Ǽ��ԡ�)��
��3��F2����NH3 �γ������ӵĽṹʽΪ ����ij����ɫ��Һ�м��백ˮ���γ���ɫ�������������백ˮ���������ܽ�����ɫ����Һ���ɵõ��������������ӵ�����д�������ܽ�����ӷ���ʽ ��
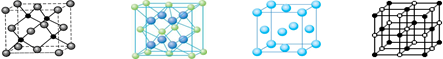
��4��A ��E����Ԫ���γɾ��徧������ͼ�е� (��١��ڡ��ۻ��)��

�� �� �� ��
��5����ͼ�������߷ֱ��ʾ��A�塢��A�塢��A�塢��A��Ԫ����̬�⻯��е�仯����ѡ��C���⻯�����ڵ����� ����n��m��x��y����

��9�֣��� S��P��Cl ��1�֣� �� Sp3 ���� ���ԣ���1�֣���3�֣�
�� [Cu(NH3)4]2+��1�֣���Cu(OH)2�� 4NH3��H2O �� [Cu(NH3)4]2+ �� 2OH-�� 4H2O ��2�֣�
�� �� ��1�֣��� n��1�֣�
�� [Cu(NH3)4]2+��1�֣���Cu(OH)2�� 4NH3��H2O �� [Cu(NH3)4]2+ �� 2OH-�� 4H2O ��2�֣�
�� �� ��1�֣��� n��1�֣�
���������F���������ܲ����ȫ��������λ�ڵ������ڣ����Ը���F����Ҫ���ϼۿ�֪��F��ͭ��E��ԭ�Ӱ뾶����ͭ�ģ�Ҳλ�ڵ������ڣ���Ҫ���ϼ��ǣ�2�ۣ�����E�Ǹơ����ݶ�����Ԫ��A��B��C��D����Ҫ���ϼۺ�ԭ�Ӱ뾶��֪��A��F��B��P��C��S��D��Cl��
��1���ǽ�����Խǿ����һ������Խ������PԪ�ص�3p������Ӵ��ڰ����״̬�����һ�����ܴ���SԪ�صģ���S��P��Cl��
��2��PH3������Pԭ�Ӻ��У�5��1��3����2��1�Թ¶Ե��ӣ������������νṹ�����ڼ��Է��ӣ�Pԭ����sp3�ӻ���
��3��ͭ���ӺͰ������γ���λ�����仯ѧʽ�� [Cu(NH3)4]2+������ͭ���ӺͰ������γ���λ��������������ͭ���ܽ��ڰ�ˮ�У���Ӧ�����ӷ���ʽ��Cu(OH)2�� 4NH3��H2O �� [Cu(NH3)4]2+ �� 2OH-�� 4H2O��
��4��CaF2�γɵľ��������Ӿ��壬�侧���Ǣڣ���ѡ�ڡ�
��5������ˮ���Ӽ������������۷е���ߣ����Է���S���⻯�������Ӧ����n��
�����������ԡ����ڱ���Ԫ�ص��ƶϡ�Ϊ���壬����ѧ����Ԫ�����ڱ�����Ϥ�̶ȼ���Ա��и�Ԫ�����ʺ���Ӧԭ�ӽṹ�������Եݱ���ɵ���ʶ�����ճ̶ȡ�������ѧ�������ʽṹ�����ʹ�ϵ�Լ�����Ԫ�������ɽ�����廯ѧ�����������

��ϰ��ϵ�д�
�����Ŀ


 ��Ԫ�صĺ�������Ų�ʽ_______________________________________;
��Ԫ�صĺ�������Ų�ʽ_______________________________________; ��һ�����ܵĴ�С��ϵΪ________________(��Ԫ�ط��ű�ʾ)
��һ�����ܵĴ�С��ϵΪ________________(��Ԫ�ط��ű�ʾ)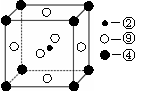

 Ti+2MgCl2
Ti+2MgCl2