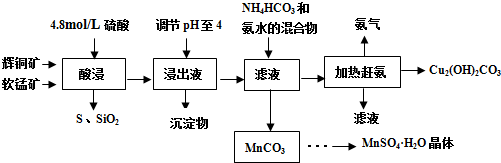
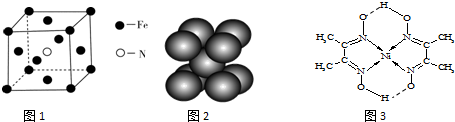
��Ŀ����
6��ijС����CoCl2•6H2O��NH4Cl��H2O2��Ũ��ˮΪԭ�ϣ��ڻ���̿���£��ϳ��˳Ȼ�ɫ����X��Ϊȷ������ɣ���������ʵ�飮�ٰ��IJⶨ����ȷ��ȡw g X��������ˮ�ܽ⣬ע����ͼ��ʾ������ƿ�У�Ȼ����μ�������10%NaOH��Һ��ͨ��ˮ����������ƷҺ�еİ�ȫ����������V1 mL cl mol•L-1���������Һ���գ�����������ȡ�½���ƿ����c2 mol•L-1NaOH����Һ�ζ���ʣ��HCl�����յ�ʱ����V2 mL NaOH��Һ��
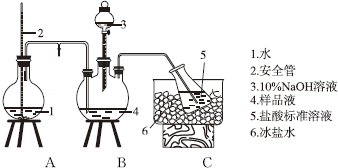
���IJⶨװ�ã���ʡ�Լ��Ⱥͼг�װ�ã�
���ȵIJⶨ��ȷ��ȡ��ƷX�������Һ����AgNO3����Һ�ζ���K2CrO4��ҺΪָʾ���������ֵ���ɫ����������ʧΪ�յ㣨Ag2CrO4Ϊש��ɫ����
�ش��������⣺
��1��װ���а�ȫ�ܵ�����ԭ���ǵ�A��ѹ������ʱ����ȫ����Һ��������ʹAƿ��ѹ���ȶ���
��2����NaOH����Һ�ζ���ʣ��HClʱ��Ӧʹ�ü�ʽ�ζ��ܣ���ʹ�õ�ָʾ��Ϊ��̪������죩��
��3����Ʒ�а���������������ʽΪ$\frac{��{C}_{1}{V}_{1}-{C}_{2}{V}_{2}����1{0}^{-3}mol��17g/mol}{wg}$��100%��
��4���ⶨ��ǰӦ�ö�װ�ý��������Լ��飬�������Բ��òⶨ�����ƫ�ͣ��ƫ�ߡ���ƫ�͡�����
��5���ⶨ�ȵĹ����У�ʹ����ɫ�ζ��ܵ�ԭ���Ƿ�ֹ����������ֽ⣻�ζ��յ�ʱ������Һ��c��Ag+��=2.0��10-5 mol•L-1��c��CrO42-��Ϊ2.8��10-3mol•L-1����֪��Ksp��Ag2CrO4��=1.12��10-12
��6�����ⶨ����ƷX���ܡ������ȵ����ʵ���֮��Ϊ1��6��3���ܵĻ��ϼ�+3���Ʊ�X�Ļ�ѧ����ʽΪ2CoCl2+2NH4Cl+10NH3+H2O2�T2[Co��NH3��6]Cl3+2H2O��X���Ʊ��������¶Ȳ��ܹ��ߵ�ԭ�����¶ȹ��߹�������ֽ⡢�����ݳ���������ɲ��������ȷ��
���� ��ȷ��ȡw g X��������ˮ�ܽ⣬ע����ͼ��ʾ������ƿ�У�Ȼ����μ�������10%NaOH��Һ��ͨ��ˮ����������ƷҺ�еİ�ȫ����������V1 mL cl mol•L-1���������Һ���գ�����������ȡ�½���ƿ����c2 mol•L-1NaOH����Һ�ζ���ʣ��HCl�����յ�ʱ����V2 mL NaOH��Һ�����а��IJⶨ��
��1��ͨ��2��Һ�����A��ѹǿ��
��2����ֻ��ʢ���ڼ�ʽ�ζ����У�������Һֻ��ʢ������ʽ�ζ����У�NaOH��Һ��������Һǡ�÷�Ӧ������ԣ�����ѡ�����Ի���Ա�ɫ��Χ�ڵ�ָʾ����
��3�����ݰ���������ᷴӦ֮��Ĺ�ϵʽ���㰱�����������ٸ�������������ʽ���㰱����������
��4���������Բ��ã����°�������ƫ�ͣ�
��5�����������ȶ��������ֽ⣻�������ӻ���������c��CrO42-����
��6�����ⶨ����ƷX���ܡ������ȵ����ʵ���֮��Ϊ1��6��3�����仯ѧʽΪ[Co��NH3��6]Cl3�����ݻ������и�Ԫ�ػ��ϼ۵Ĵ�����Ϊ0����CoԪ�ػ��ϼۣ��÷�Ӧ��Coʧ���ӡ�˫��ˮ�õ��ӣ�CoCl2•6H2O��NH4Cl��H2O2��NH3������Ӧ����[Co��NH3��6]Cl3��ˮ��˫��ˮ�ֽ⡢������ܽ�������¶ȵ����߶����ͣ�
��� �⣺��1����������ƿ��ѹǿ������С�����������Σ�գ�������A�ڵ�����Һ�����ߣ�������ѹ��������С��������ͨ�����ܽ�����ƿ��Ҳ������ɵ�������ȫ���õ�ԭ����ʹA��ѹǿ�ȶ���
�ʴ�Ϊ����A��ѹ������ʱ����ȫ����Һ��������ʹAƿ��ѹ���ȶ���
��2����ֻ��ʢ���ڼ�ʽ�ζ����У�������Һֻ��ʢ������ʽ�ζ����У�������NaOH����Һȷ����ʣ��HClʱ��Ӧʹ�ü�ʽ�ζ���ʢ��NaOH��Һ��NaOH��Һ��������Һǡ�÷�Ӧ������ԣ�����ѡ�����Ի���Ա�ɫ��Χ�ڵ�ָʾ��������Ϊ���Ա�ɫָʾ������̪Ϊ���Ա�ɫָʾ�������Կ���ѡȡ���Ȼ��̪��ָʾ����
�ʴ�Ϊ�����̪����ȣ�
��3���백����Ӧ��n��HCl��=V1��10-3L��C1mol•L-1-C2mol•L-1 ��V2��10-3L=��C1V1-C2V2����10-3mol�����ݰ�����HCl�Ĺ�ϵʽ֪��n��NH3��=n��HCl��=��C1V1-C2V2����10-3mol��������������=$\frac{��{C}_{1}{V}_{1}-{C}_{2}{V}_{2}����1{0}^{-3}mol��17g/mol}{wg}$��100%��
�ʴ�Ϊ��$\frac{��{C}_{1}{V}_{1}-{C}_{2}{V}_{2}����1{0}^{-3}mol��17g/mol}{wg}$��100%��
��4���������Բ��ã����²��ְ���й©����������������ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
��5�����������ȶ��������ֽ⣬Ϊ��ֹ�������ֽ⣬����ɫ�ζ���ʢ����������Һ��c��CrO42-��=$\frac{Ksp}{{c}^{2}��A{g}^{+}��}$=$\frac{1.12��1{0}^{-12}}{��2.0��1{0}^{-5}��^{2}}$mol/L=2.8��10-3 mol/L��
�ʴ�Ϊ����ֹ����������ֽ⣻2.8��10-3��
��6�����ⶨ����ƷX���ܡ������ȵ����ʵ���֮��Ϊ1��6��3�����仯ѧʽΪ[Co��NH3��6]Cl3�����ݻ������и�Ԫ�ػ��ϼ۵Ĵ�����Ϊ0��CoԪ�ػ��ϼ�Ϊ+3�ۣ��÷�Ӧ��Coʧ���ӡ�˫��ˮ�õ��ӣ�CoCl2•6H2O��NH4Cl��H2O2��NH3������Ӧ����[Co��NH3��6]Cl3��ˮ����Ӧ����ʽΪ2CoCl2+2NH4Cl+10NH3+H2O2$\frac{\underline{\;����\;}}{\;}$2[Co��NH3��6]Cl3+2H2O��˫��ˮ�ֽ⡢������ܽ�������¶ȵ����߶����ͣ�����X���Ʊ��������¶Ȳ��ܹ��ߣ�
�ʴ�Ϊ��+3��2CoCl2+2NH4Cl+10NH3+H2O2$\frac{\underline{\;����\;}}{\;}$2[Co��NH3��6]Cl3+2H2O���¶�Խ�߹�������ֽ⡢�����ݳ���������ɲ��������ȷ��
���� ���⿼�������ʺ����IJⶨ���漰��������ܽ�ƽ�⡢������ԭ��Ӧ�����ʺ����IJⶨ��֪ʶ�㣬��ȷʵ��ԭ���ǽⱾ��ؼ���֪��ָʾ����ѡȡ��������Ŀ�Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д�| A�� | ���������ƹ��������ֽ�ϳ��� | |
| B�� | ��10mL��Ͳ��ȡ8.58mL����ˮ | |
| C�� | ��ȡ����ʱ���ö���������Ũ�����ڳ����·�Ӧ��������ˮ�������ռ� | |
| D�� | ����ʱ��������ĩ��Ӧ������������ֽ�� |
| A�� |  ������ƿ��ת����Һ | B�� |  �к��ȵIJⶨ | ||
| C�� | 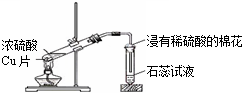 Ũ������ͭ�ķ�Ӧ | D�� |  �Ҷ��ᾧ�����ȷֽ� |
| A�� | MnO2��Mn2+ | B�� | PCl3��PCl5 | C�� | HCl��H2 | D�� | H2O2��O2 |
| A�� | ���Ƶ���ˮ�ڹ�����������ɫ��dz | |
| B�� | ��ѹ�Ժϳɰ����� | |
| C�� | ��NO2��N2O4�Ļ�����彵�º�������ɫ��dz | |
| D�� | ���������ʹ�ϳɰ������ʼӿ� |