��Ŀ����
16����ͭ����Ҫ�ɷ�Cu2S�������������SiO2��Fe2O3�����ʣ����̿���Ҫ���� MnO2���Լ�����SiO2��Fe2O3�����ʣ��о���Ա�����ۺ�������������Դ����ͬ�����ʪ��ұ�����գ��Ʊ������̺ͼ�ʽ̼��ͭ����Ҫ�����������£�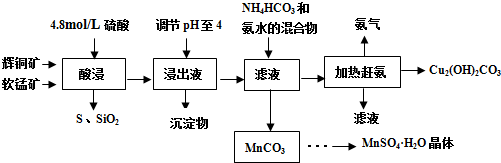
��֪����MnO2�н�ǿ�������ԣ��ܽ�Cu+������Cu2+
��[Cu��NH3��4]SO4�����ȶ�������ˮ��Һ�л�ֽ�����NH3
�۲��ֽ��������������������������pH��Χ����ʼ��������ȫ������pH����
Fe3+��1.5��3.2 Mn2+��8.3��9.8 Cu2+��4.4��6.4
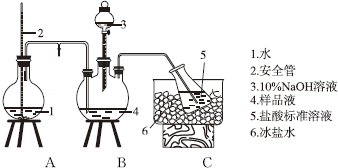
��1��ʵ��������250mL 4.8mol/L��ϡ���ᣬ����IJ�������������������Ͳ���ձ������Ҫ250mL����ƿ����ͷ�ι�
��2�����ʱ��Ϊ����߽�ȡ�ʿɲ�ȡ�Ĵ�ʩ�з����ʯ���ʵ������¶Ȼ���裨��дһ�㣩��
��3�����ʱ���õ�����Һ����Ҫ����CuSO4��MnSO4�ȣ�д���÷�Ӧ�Ļ�ѧ����ʽCu2S+2MnO2+4H2SO4=2CuSO4+2MnSO4+S��+4H2O��
��4�����ڽ���ҺpH=4��������ʹFe3+��ȫ����Fe��OH��3������
��5���������п�ѭ��ʹ�õ������ǣ�д��ѧʽ��NH3��
��6����õ�MnSO4•H2O������þƾ�ϴ�ӣ��ŵ��Ǽ���MnSO4•H2O�������ʧ��
��7���ñ���BaCl2��Һ�ⶨ��Ʒ��MnSO4•H2O��������ʱ��������Ʒ���ȴ���100%���ⶨ�����в��������ɺ��ԣ��������ԭ���л������������ʻ־���ʧȥ�ᾧˮ ����дһ�֣���
���� ���̷�����֪��ͭ�����̿��������������˵õ�����Һ������ҺPH��ʹ������ȫ�����������˵õ�����Һ����Ҫ����CuSO4��MnSO4�ȣ�����̼����狀Ͱ�ˮ���˵õ������Һ���ȸϳ������ᾧ�����õ���ʽ̼��ͭ������Ϊ̼���̣�ͨ������Ũ������ȴ�ᾧ����ϴ�ӵõ������̾��壻
��1������250mL��Һ����Ҫ250mL����ƿ������Ҫ��ͷ�ιܶ��ݣ�
��2����ʯ�Ŀ�����С���¶ȼ��Ƿ����Ȼ�Ӱ���ȡ�ʣ�
��3��MnO2�н�ǿ�������ԣ��ܽ����������е�������Ϊ��������������CuSO4��MnSO4��S����Ӧ�������ᡢ�������̺�����ͭ�����ݻ��ϼ���������ƽ��
��4������������ʼ����PH=1.5����ȫ����PH=3.2��Cu2+����ʼ������4.4��ʹFe3+��ȫ������Cu2+��������PH��Χ�ǣ�3.2��4.4��
��5�����ݹ������̿��Կ�������ѭ��ʹ�õ������ǰ�����
��6��MnSO4•H2O�����ھƾ����ܽ��С��
��7������ʧȥ�ᾧˮ���ⶨ�����̾�����������������100%��
��� �⣺��1������һ�����ʵ���Ũ�ȵ���Һ���ز����ٵ�������ƿ�ͽ�ͷ�ιܣ�����ƿҪ������������ʹ��250mL����ƿ��
�ʴ�Ϊ��250mL����ƿ����ͷ�ιܣ�
��2���������ʱ��ʯ������СӰ���ȡ�ʣ�����ͨ�������ʯ��߽�ȡ�ʣ��������ʵ������¶Ȼ��߽�����߽�ȡ�ʣ�
�ʴ�Ϊ�������ʯ���ʵ������¶Ȼ��߽��裻
��3��������Ϣ��MnO2�ܽ����������е�������Ϊ������Ӧ����Cu2S��MnO2��H2SO4 ����������CuSO4��MnSO4��S��
������ͭ�������壬���ϼ۱仯�ǣ���1��2+2��1��=4��
MnԪ�ػ��ϼ۱仯�ǣ�4-2=2��
���Զ������̻�ѧ��������2������ͭ����1���ٸ��ݹ۲취��ƽ�������ʣ���Ӧ�Ļ�ѧ����ʽ�ǣ�Cu2S+2MnO2+4H2SO4=2CuSO4+2 MnSO4+S��+4H2O��
�ʴ�Ϊ��Cu2S+2MnO2+4H2SO4=2CuSO4+2 MnSO4+S��+4H2O��
��4�����ݽ��������������������������pH��Χ��ʹFe3+��ȫ������Cu2+��������PH��Χ�ǣ�3.2��4.4����ѡ��PH=4Ŀ����ʹFe3+��ȫˮ��������������������
�ʴ�Ϊ��ʹFe3+��ȫ����Fe��OH��3������
��5�����������У������˰�ˮ������ֵõ��˰�����NH3 ��ѭ��ʹ�ã��ʴ�Ϊ��NH3 ��
��6���ھƾ��У������̾����ܽ��С���ʻ�õ�MnSO4•H2O������þƾ�ϴ�ӣ�Ŀ���Ǽ���MnSO4•H2O�������ʧ��
�ʴ�Ϊ������MnSO4•H2O�������ʧ��
��7��������������100%��˵���������̾����п��ܻ������������ʻ־���ʧȥ�ᾧˮ���ʴ�Ϊ���������������ʻ־���ʧȥ�ᾧˮ��
���� ���⿼���������̾������ȡ���漰��֪ʶ��Ƚ϶࣬���Ը�����ѧ֪ʶ�����������ó���ȷ���ۣ������Ѷ��еȣ�

 ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�| A�� | 12 | B�� | 11 | C�� | 7 | D�� | 3 |
| A�� | ��ͬ��ͬѹ�£���ͬ������κ�����������ԭ������ͬ | |
| B�� | ��ͬ�����£�N2��O2�Ļ��������������N2����ԭ������� | |
| C�� | �����ʵ�����NH4+��OH-��������������ҵ�����Ҳ��� | |
| D�� | 1mol �һ��к��еĵ�����Ϊ18NA |
��1���±��г������������Ħ���������ڱ�����д����Ħ�����������������
| ���� | �������ͯ���� | �������������� | �л������� |
| Ħ���� | �������� | ̼��� | �������� |
| Ħ�������������ָ�ᡢ��Ρ����������������� |
��3�������е�Ħ����̼��ƿ�����ʯ��ʯ���Ʊ���ijѧ�������һ��ʵ�����Ʊ�̼��Ƶ�ʵ�鷽����������ͼΪ��[ʯ��ʯ]$\stackrel{�ٸ���}{��}$[��ʯ��]$\stackrel{�ڼ�ˮ}{��}$[ʯ��ˮ]$\stackrel{�ۼ�Na_{2}CO_{3}��Һ}{��}$[̼���]
��д�������������йط�Ӧ�Ļ�ѧ����ʽ����ע����Ӧ���ͣ�
��CaCO3$\frac{\underline{\;����\;}}{\;}$CO2��+CaO���ֽⷴӦ��
��CaO+H2O�TCa��OH��2�����Ϸ�Ӧ��
��Ca��OH��2+Na2CO3�TCaCO3��+2NaOH�����ֽⷴӦ��
��4����������ʯ��ʯ��ԭ�ϣ������Լ���ѡ�������ʵ�����Ʊ�̼��Ƶ���һ��ʵ�鷽�������գ�3����ʾ�������ʵ�鷽��������ͼ��ʾ������
ʯ��ʯ-��
����Ƶķ������ŵ�Ϊ����Ӧ���������ڲ��������ò�Ʒ���ȸߣ�
��5�������������Ƿ���̼��Ƶ�ʵ�鷽���ǣ�ȡ������Ʒ������ϡ���ᣬ�۲�����ʹ����ʯ��ˮ����ǵ�������������У���CaCO3��������
| A�� | ���³�ѹ�£�11.2LCO2��8.5gNH3������������� | |
| B�� | �����ʵ�����H3O+��OH-������֮���������֮����ͬ | |
| C�� | ���³�ѹ�£�48gO3��O2�Ļ�������к��е���ԭ����Ϊ3NA | |
| D�� | ��״���£�22.4LH2��Cl2�Ļ�������к��еķ�����Ϊ2NA |
| A�� | ��ͬ��Ԫ����ɵ�����һ�����ڴ����� | |
| B�� | NaHCO3��ˮ�е��룺NaHCO3�TNa++H++CO32- | |
| C�� | ������Ԫ�ص����Ӳ�һ������������ | |
| D�� | ������ͬ����������������ͬ��Ԫ�� |