��Ŀ����
����Ŀ����Դ����ϡ���Ϣһ�𱻳�Ϊ�ִ���ᷢչ������֧���������Դ�ݽߵ�Σ���������Դ�����ʺͿ�������Դ�ǽ����һ�����������Ҫ����
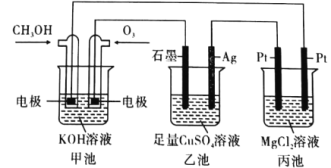
(1)��ѧ��Ӧ���ʺ���������������������أ����ǻ�ѧѧ�ƹ�ע�ķ���֮һ��ijͬѧΪ��̽��п�����ᷴӦ�����е����ʱ仯���� 400mL ϡ�����м���������п�ۣ�����ˮ���ռ���Ӧ�ų���������ʵ���¼����(�ۼ�ֵ):
ʱ��/min | 1 | 2 | 3 | 4 | 5 |
�������/mL(���) | 100 | 240 | 464 | 576 | 620 |
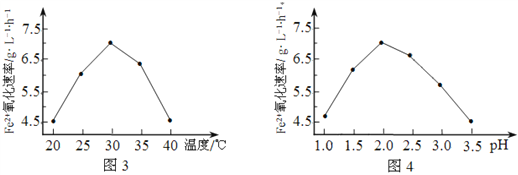
����һ��ʱ���ڷ�Ӧ�������____min(����0��1������1��2������2��3������3��4�� ����4��5��)��
����һѧ��Ϊ���Ʒ�Ӧ���ʷ�ֹ��Ӧ�������Բ�������������������������м���������������Һ�Լ�����Ӧ���ʵ���Ӱ��������������������Ϊ���е���____(����ĸ���)��
A.KCl ��Һ B.Ũ���� C.����ˮ D.CuSO4 ��Һ
(2)��ͼΪԭ���װ��ʾ��ͼ��
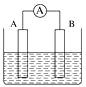
���� A Ϊ����B Ϊþ�������Ϊϡ������Һ����������_______(������Ƭ������þƬ��)��
����AΪCu��B Ϊʯī�������Ϊ FeCl3 ��Һ������ʱ���ܷ�ӦΪ 2FeCl3+Cu=2FeCl2+CuCl2��д��ͭ�缫�ĵ缫��Ӧʽ��____�����õ�ط�Ӧ������ 0.1mol FeCl3�� ��ת�Ƶ��ӵ���ĿΪ _____��
���𰸡�2��3 AC þƬ Cu-2e-=Cu2+ 0.1NA
��������
(1)����Ӧ����Խ�죬��ͬʱ�����ռ�������Խ�ࣻ
��Ҫ���ͷ�Ӧ���ʣ����Բ��ý���������Ũ�ȵķ���ʵ�֣�
(2)������Ƭ��þƬ�õ��������������Ϊϡ������Һ��þ�������ã�þ��������
����AΪͭƬ��BΪʯī�������ΪFeCl3��Һ��Cuʧ������������ʯī��������������ͭʧ��������ͭ���ӡ�ʹ����Cu��������С�������������ӵõ��������������ӣ���ϵ缫��Ӧʽ����ת�Ƶ�����Ŀ��
(1)����ͬ�����£���Ӧ����Խ����ͬʱ�����ռ�������Խ�࣬0��1 min�ռ�����100 mL��1��2 min�ռ�����140 mL��2��3 min�ռ�����224 mL��3��4 min�ռ�����112 mL��4��5 min�ռ�����44 mL���ɴ˿ɼ���2��3 min�ռ����������࣬��2��3 minʱ���ڷ�Ӧ�������
��A������KCl��Һ��ʹ��Һ��c(H+)���ͣ���Ӧ���ʽ��ͣ��������������ʵ������䣬���������������䣬A�������⣻
B������Ũ������Һ��ʹ��Һ��c(H+)����ѧ��Ӧ���������������������ʵ������ӣ�ʹ����������������B���������⣻
C����������ˮ��ʹ��Һ��c(H+)��С����Ӧ���ʽ��ͣ��������������ʵ������䣬���������������䣬C�������⣻
D������CuSO4��Һ��Zn��ͭ���ӷ����û���Ӧ����Cu��Zn���û�������Cu��ϡ���ṹ����ԭ��ض��ӿ췴Ӧ���ʣ�D���������⣻
�ʺ���ѡ����AC��
(2)���� A Ϊ����B Ϊþ�������Ϊϡ������Һ����þ�������ã���������þƬ��
����AΪCu��B Ϊʯī�������Ϊ FeCl3 ��Һ������ʱ���ܷ�ӦΪ 2FeCl3+Cu=2FeCl2+CuCl2�����ݷ���ʽ��֪��CuΪ������Cuʧ��������ͭ���ӣ�ͭ�缫�ĵ缫��ӦʽΪCu-2e-=Cu2+���������ϣ���Һ�е�Fe3+�õ�������Fe2+��������B�ĵ缫��ӦʽΪFe3++e-=Fe2+�����������ĵ缫��Ӧʽ��֪�����õ�ط�Ӧ������ 0.1mol FeCl3ʱ��ת�Ƶ��ӵ����ʵ���Ϊ0.1mol����ĿΪ0.1NA��

 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�����Ŀ���黯��(GaAs)������������ͨ���ݵ�100���������ܼ�Ϊ10%���ƹ��黯��(GaAs)�� ������(GaN)�ȷ�������ܣ�LED���������ǽ��ܼ��ŵ���Ч�ٴ롣��ش��������⣺
(1)��̬Nԭ�Ӻ�������Ų�ͼΪ _____________________ ��Ga��Alͬ���壬��λ��Al����һ���ڣ����̬Gaԭ�ӵļ۵����Ų�ʽΪ ________________��
(2)Ga�ĵ縺�Ա�As______��������������С������Ga��ʧȥ���ӵĵ����� ����λ��kJ��mol-1������ֵ����Ϊ577��1985��2962��6192���ɴ˿���֪Ga����Ҫ���ϼ�Ϊ__��+3��
(3)�Ƚ�����Ga��±������۵�ͷе㣬 GaCl3��GaBr3��GaI3���ۡ��е���������, ������仯��ԭ���ǣ�_____________________________________________________��
�ص�±���� | GaCl3 | GaBr3 | GaI3 |
�۵�/�� | 77.75 | 122.3 | 211.5 |
�е�/�� | 201.2 | 279 | 346 |
GaF3���۵㳬��1000�棬���ܵ�ԭ����____________________________��
(4)GaAs�ǽ�(CH3)3Ga��AsH3�ý����л��ﻯѧ������������Ʊ��õ����÷�Ӧ��700���½���
����÷�Ӧ�Ļ�ѧ����ʽΪ��____________________________________________��
�ڷ�Ӧ��AsH3���ӵļ��ι���Ϊ________________��(CH3)3Ga����ԭ���ӻ���ʽΪ____________________��