��Ŀ����
����Ŀ���黯��(GaAs)������������ͨ���ݵ�100���������ܼ�Ϊ10%���ƹ��黯��(GaAs)�� ������(GaN)�ȷ�������ܣ�LED���������ǽ��ܼ��ŵ���Ч�ٴ롣��ش��������⣺
(1)��̬Nԭ�Ӻ�������Ų�ͼΪ _____________________ ��Ga��Alͬ���壬��λ��Al����һ���ڣ����̬Gaԭ�ӵļ۵����Ų�ʽΪ ________________��
(2)Ga�ĵ縺�Ա�As______��������������С������Ga��ʧȥ���ӵĵ����� ����λ��kJ��mol-1������ֵ����Ϊ577��1985��2962��6192���ɴ˿���֪Ga����Ҫ���ϼ�Ϊ__��+3��
(3)�Ƚ�����Ga��±������۵�ͷе㣬 GaCl3��GaBr3��GaI3���ۡ��е���������, ������仯��ԭ���ǣ�_____________________________________________________��
�ص�±���� | GaCl3 | GaBr3 | GaI3 |
�۵�/�� | 77.75 | 122.3 | 211.5 |
�е�/�� | 201.2 | 279 | 346 |
GaF3���۵㳬��1000�棬���ܵ�ԭ����____________________________��
(4)GaAs�ǽ�(CH3)3Ga��AsH3�ý����л��ﻯѧ������������Ʊ��õ����÷�Ӧ��700���½���
����÷�Ӧ�Ļ�ѧ����ʽΪ��____________________________________________��
�ڷ�Ӧ��AsH3���ӵļ��ι���Ϊ________________��(CH3)3Ga����ԭ���ӻ���ʽΪ____________________��
���𰸡�![]() 4s24p1 С +1 GaCl3��GaBr3��GaI3��Ϊ���Ӿ��壬�ṹ���ƣ���Է����������������Ӽ�������������ǿ GaF3Ϊ���Ӿ��� (CH3)3Ga + AsH3
4s24p1 С +1 GaCl3��GaBr3��GaI3��Ϊ���Ӿ��壬�ṹ���ƣ���Է����������������Ӽ�������������ǿ GaF3Ϊ���Ӿ��� (CH3)3Ga + AsH3![]() GaAs + 3CH4 ������ sp2
GaAs + 3CH4 ������ sp2
��������
��1��Nԭ�Ӻ�����7�����ӣ���������Ų�ʽΪ1s22s22p3��ͬ����Ԫ��������������ͬ��
��2��ͬ����Ԫ�أ�������Ԫ�طǽ������������縺����������������ĸ������ܵ����ݿ�֪����һ�͵ڶ��������͵��ĵ����ܲ��ϴ�
��3���ṹ���Ƶķ��Ӿ��壬��Է�������Խ���Ӽ�������Խǿ���۵�ͷе�Խ�ߣ����Ӿ�����нϸߵ��۵�ͷе㣻
��4������700��ʱ����CH3��3Ga��AsH3��Ӧ����GaAs�ͼ��飻
��AsH3�����к���3���Ҽ���1���µ��Ӷԣ���CH3��3Ga��Gaԭ���γ�3���Ҽ���û�йµ��Ӷԡ�
��1��Nԭ�Ӻ�����7�����ӣ���������Ų�ʽΪ1s22s22p3�����������Ų�ͼΪ![]() ��ͬ����Ԫ��������������ͬ��Ga��Alͬ���壬Al�ļ۵����Ų�ʽΪ3s23p1������һ����Gaԭ�ӵļ۵����Ų�ʽΪ4s24p1���ʴ�Ϊ��
��ͬ����Ԫ��������������ͬ��Ga��Alͬ���壬Al�ļ۵����Ų�ʽΪ3s23p1������һ����Gaԭ�ӵļ۵����Ų�ʽΪ4s24p1���ʴ�Ϊ��![]() ��4s24p1��
��4s24p1��
��2��ͬ����Ԫ�أ�������Ԫ�طǽ������������縺������������Ga�ĵ縺�Ա�AsС��������ĸ������ܵ����ݿ�֪����һ�͵ڶ��������͵��ĵ����ܲ��ϴ���Ga����Ҫ���ϼ�Ϊ+1��+3���ʴ�Ϊ��С��+1��
��3���ṹ���Ƶķ��Ӿ��壬��Է�������Խ���Ӽ�������Խǿ���۵�ͷе�Խ�ߣ��������±������۵�ͷе��֪��GaCl3��GaBr3��GaI3Ϊ�ṹ���Ƶķ��Ӿ��壬��GaBr3��GaI3����Է����������������Ӽ�������������ǿ���۵�ͷе��������ߣ����Ӿ�����нϸߵ��۵�ͷе㣬��GaF3���۵㳬��1000����֪��GaF3Ϊ���Ӿ��壬�ʴ�Ϊ��GaCl3��GaBr3��GaI3��Ϊ���Ӿ��壬�ṹ���ƣ���Է����������������Ӽ�������������ǿ��GaF3Ϊ���Ӿ��壻
��4������700��ʱ����CH3��3Ga��AsH3��Ӧ����GaAs�ͼ��飬��Ӧ�Ļ�ѧ����ʽΪ(CH3)3Ga + AsH3 ![]() GaAs + 3CH4���ʴ�Ϊ��(CH3)3Ga + AsH3
GaAs + 3CH4���ʴ�Ϊ��(CH3)3Ga + AsH3 ![]() GaAs + 3CH4��
GaAs + 3CH4��
��AsH3�����к���3���Ҽ���1���µ��Ӷԣ��ռ乹��Ϊ�����Σ���CH3��3Ga��Gaԭ���γ�3���Ҽ���û�йµ��Ӷԣ��ӻ���ʽΪsp2�ӻ����ʴ�Ϊ�������Σ�sp2��

 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д� �������ϵ�д�
�������ϵ�д�����Ŀ����Դ����ϡ���Ϣһ�𱻳�Ϊ�ִ���ᷢչ������֧���������Դ�ݽߵ�Σ���������Դ�����ʺͿ�������Դ�ǽ����һ�����������Ҫ����
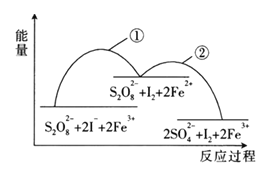
(1)��ѧ��Ӧ���ʺ���������������������أ����ǻ�ѧѧ�ƹ�ע�ķ���֮һ��ijͬѧΪ��̽��п�����ᷴӦ�����е����ʱ仯���� 400mL ϡ�����м���������п�ۣ�����ˮ���ռ���Ӧ�ų���������ʵ���¼����(�ۼ�ֵ):
ʱ��/min | 1 | 2 | 3 | 4 | 5 |
�������/mL(���) | 100 | 240 | 464 | 576 | 620 |
����һ��ʱ���ڷ�Ӧ�������____min(����0��1������1��2������2��3������3��4�� ����4��5��)��
����һѧ��Ϊ���Ʒ�Ӧ���ʷ�ֹ��Ӧ�������Բ�������������������������м���������������Һ�Լ�����Ӧ���ʵ���Ӱ��������������������Ϊ���е���____(����ĸ���)��
A.KCl ��Һ B.Ũ���� C.����ˮ D.CuSO4 ��Һ
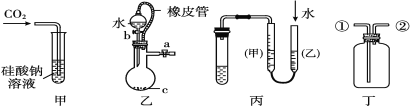
(2)��ͼΪԭ���װ��ʾ��ͼ��
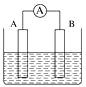
���� A Ϊ����B Ϊþ�������Ϊϡ������Һ����������_______(������Ƭ������þƬ��)��
����AΪCu��B Ϊʯī�������Ϊ FeCl3 ��Һ������ʱ���ܷ�ӦΪ 2FeCl3+Cu=2FeCl2+CuCl2��д��ͭ�缫�ĵ缫��Ӧʽ��____�����õ�ط�Ӧ������ 0.1mol FeCl3�� ��ת�Ƶ��ӵ���ĿΪ _____��
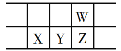
����Ŀ���±���Ԫ�����ڱ���ǰ�����ڣ���ش��й����⣺�����������þ��廯ѧ����ش�
1 | �� | |||||||
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | ||||
4 | �� | �� | ||||||
��1���ܵ�ԭ�ӽṹʾ��ͼΪ_________������Ԫ�����ڱ�λ��________������Ԫ��������������ˮ����������ǿ����_________������ˮ���ﻯѧʽ��
��2���ݵ�����������ˮ����͢���������ﷴӦ�����ӷ���ʽ________________
��3���õ���ʽ��ʾԪ�آٺ͢��γɵĻ�������γɹ���________________
��4���ݵ����������ˮ����ĵ���ʽ________________
��5�������ʵ��֤��������������ǿ��________________