��Ŀ����
����Ŀ��ijѧ������ͼװ�ý���CO��CO2�������ķ���������aΪ���ɼ�(��������ͨ��)��bΪ��Һ©���Ļ���(�û������������ڿ��Ʒ�Һ©����Һ����������)��
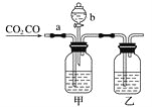
(1)���������ơ�ϡ���ᡢŨ���Ἰ��������ѡ����ʵ������������пո��У���ɴﵽʵ��Ŀ�ģ���ƿ��ʢ________��Һ����ƿ��ʢ________��Һ����Һ©����ʢ________��Һ��
(2)ʵ��ʱ�ȷ����CO���������ȹر�___(��a��b����ͬ)����___��д����ʱ������Ӧ�����ӷ���ʽ��____��
(3)������CO�����ռ�CO2���������ȹر�____���ٴ�____��д����ʱ����CO2��Ӧ�����ӷ���ʽ��__��
���𰸡��������� Ũ���� ϡ���� b a 2OH����CO2=CO32����H2O a b CO32����2H��=CO2����H2O
��������
����ʵ��Ŀ�ļ�װ���ص㣬������NaOH��Һ���ն�����̼���ռ�CO��������ϡ������̼���Ʒ�Ӧ���ɶ�����̼�����ռ���
��1��������֪����װ��ʢ��NaOH��Һ����Һ©����ʢ�е�Ϊϡ���ᣬװ����ʢ�е�ΪŨ���ᡣ
(2)ʵ��ʱ�ȷ����CO�����ն�����̼��Ӧ�ر�b����a��������̼��NaOH��Ӧ����̼���ƺ�ˮ����Ӧ�����ӷ���ʽΪ2OH����CO2=CO32����H2O��
(3)������CO��������ϡ������̼���Ʒ�Ӧ���ɲ��ռ�CO2��Ӧ��b���ر�a�����������ӷ���ʽΪCO32����2H��=CO2����H2O��

����Ŀ������ͼ��ʾװ�ý�������ʵ�飬��������Һ������У�Ԥ���������ʵ���������

ѡ�� | �������� | �������� | Ԥ����е����� |
A | Ũ���� | ��ɰֽ��ĥ������Ƭ | ������������ |
B | AgNO3��Һ | Ũ��ˮ | ������ɫ�����Ҳ���ʧ |
C | NH4ClŨ��Һ | Fe��C����� | ��Һ�������ݲ��� |
D | H2O2��Һ | FeCl3��Һ | ��Һ����ɫ |
A. AB. BC. CD. D