��Ŀ����
��8�֣���ú��Ϊȼ�Ͽ�ͨ����������;����
;��I��C(s) +O2 (g)=CO2(g) ��H1<0 ��
;��II�����Ƴ�ˮú����C(s) +H2O(g)=CO(g)+H2(g) ��H2>0 ��
��ȼ��ˮú����2CO(g)+O2 (g)=2CO2(g) ��H3<0 ��
2H2(g)+O2 (g)=2H2O(g) ��H4<0 ��
��ش��������⣺
��1��;��I�ų������� ( ����ڡ������ڡ���С�ڡ�) ;��II�ų���������
��2����H1����H2����H3����H4����ѧ��ϵʽ�� ��
��3��12g̿���������в���ȫȼ������һ����̼���ų�110.35kJ���������Ȼ�ѧ����ʽΪ ��
��4��ú̿��Ϊȼ�ϲ���;��II���ŵ��� ��
��1������ ��2����H1=��H2+1/2����H3+��H4�� ��3��C(s) +!/2O2(g)=CO(g) ��H=-110.35KJ/mol ��4��ȼ��ȼ�ճ�֣������ʸߣ����ȶ࣬��Ⱦ�١�
���������������ѧ��Ӧ�����������Ķ���ֻ�����ʵ���ʼ״̬������״̬�йأ����뷴Ӧ;���ء������ʼ״̬������״̬��ͬ����ų�������Ҳ����ͬ����H1=��H2+1/2����H3+��H4��.12g ̿���������в���ȫȼ������һ����̼���ų�110.35kJ���������Ȼ�ѧ����ʽΪ��C(s) +!/2O2(g)=CO(g) ��H=-110.35KJ/mol��ú̿��Ϊȼ�ϲ���;��II����ʹȼ�ϳ��ȼ�գ�ȼ�ϵ������ʸߣ��ų������࣬��ȾҲ�١���������
���㣺�����˹���ɵ�Ӧ�á�

��1�� 8gҺ̬��CH3OH����������ȫȼ�գ����ɶ�����̼�����Һ̬ˮʱ�ͷų�Q kJ����������д��Һ̬CH3OHȼ���ȵ��Ȼ�ѧ����ʽ ��
��2���ڻ�ѧ��Ӧ�����У��ƻ��ɻ�ѧ����Ҫ�����������γ��»�ѧ���ֻ��ͷ�������
| ��ѧ�� | H��H | N��H | N��N |
| ����/kJ·mol��1 | 436 | 391 | 945 |
�Ը��ݱ������м������ݼ���a����ֵΪ�� ��
��3����֪��C(s��ʯī)��O2(g)=CO2(g) ��H1����393.5 kJ/mol
2H2(g)��O2(g)=2H2O(l) ��H2����571.6 kJ/mol
2C2H2(g)��5O2(g)=4CO2(g)��2H2O(l) ��H3����2599 kJ/mol
���ݸ�˹���ɣ���C(s��ʯī)��H2(g)����1 mol C2H2(g)��Ӧ���Ȼ�ѧ����ʽ��
��
��֪������Һ��CrO42���Ի�ɫ��Cr2O72-�ԳȺ�ɫ
��PbCrO4������ˮ��Ҳ������ǿ��
��H+(aq��+OH-��aq��=H2O(l���� ��H=" ��a" KJ/mol
3Cl2��g��+2Cr3+��aq��+16OH-(aq��=2CrO42-��aq��+6Cl-��aq��+8H2O(l)����H="��b" KJ/mol
2CrO42-��aq��+2H+(aq��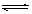 Cr2O72-��aq��+H2O(l������H="��c" KJ/mol
Cr2O72-��aq��+H2O(l������H="��c" KJ/mol
ƽ�ⳣ��K=9��5��104 ������a��b��c������0��
��������Ӧ�ݣ�ȡ50mL��Һ�������飬���ֲⶨ�������£�
| ʱ�䣨s�� | 0 | 0��01 | 0��02 | 0��03 | 0��04 |
| n ��CrO42������mol�� | 0��01 | 8��0��10-4 | 5��4��10-4 | 5��0��10-4 | |
| n ��Cr2O72������mol�� | 0 | | 4��73��10-3 | | 4��75��10-3 |
�Իش��������⣺
��1��0.02s��0.03s֮����Cr2O72-��ʾ�÷�Ӧ��ƽ����Ӧ����Ϊ
����˵����ȷ�ģ� ��
A��0.03sʱV����CrO42����=2V�棨Cr2O72����
B����ҺpHֵ����˵���÷�Ӧ�Ѵ�ƽ��״̬
C����Һ��c��CrO42������c��Cr2O72����=2:1ʱ�÷�Ӧ�Ѵ�ƽ��״̬
D����Ӧ����2��5��10-3c KJʱCrO42����ת����Ϊ50��
E�����¸÷�Ӧƽ�ⳣ�����
F��0��04sʱ����������Pb��NO3)2 ��ʹ��Һ�ɳ�ɫ��Ϊ��ɫ
��3��0��03sʱ��Һ��pH=
��4����֪����������Cr2O72����Cl-����ΪCl2����������ԭΪCr3+Ϊ���ȷ�Ӧ����д���÷�Ӧ���Ȼ�ѧ����ʽ��
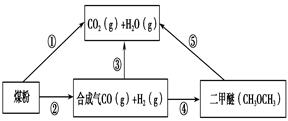
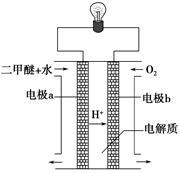
�������������ʡ�����ȵ���Ҫԭ�ϣ�Χ�ƺϳɰ����ǽ�����һϵ�е��о�
��1�����������뵪������������������Ӧ�����Ƿ�Ӧ������ȴ����ͬ��
��֪��2H2 (g) + O2 (g) = 2H2O (g) ��H =" -483.6" kJ/mol
3H2 (g) + N2 (g)  2NH3 (g) ��H =" -92.4" kJ/mol
2NH3 (g) ��H =" -92.4" kJ/mol
�������1 mol N��N����Ҫ���� kJ �� ���������л�ѧ�������������еĻ�ѧ���� ���ǿ�����������������������߷�Ӧ��������ͬ��
��2���̵��ǿ�ѧ�������о�����Ҫ���⡣��Ȼ���д�����Ȼ�Ĵ����̵����̣�N2 (g) + O2 (g) =" 2NO" (g) ��H =" +180.8" kJ/mol ����ҵ�ϳɰ������˹��̵���
�������̵ֹ���Ӧ��ƽ�ⳣ�������н�����ȷ���� ��
| ��Ӧ | �����̵� | ��ҵ�̵� | ||||
| �¶�/�� | 27 | 2000 | 25 | 350 | 400 | 450 |
| K | 3.84��10-31 | 0.1 | 5��108 | 1.847 | 0.507 | 0.152 |
A�������£������̵����������ܽ��У�����ҵ�̵��dz�������
B��������ģģ������̵����������
C����ҵ�̵��¶�Խ�ͣ�������������ӦԽ��ȫ
D��KԽ��˵���ϳɰ���Ӧ������Խ��
��3���ں��º����ܱ������а��ռס��ҡ������ַ�ʽ�ֱ�Ͷ��, ������Ӧ��3H2 (g) + N2 (g)
 2NH3 (g)��ü�������H2��ת����Ϊ40%��
2NH3 (g)��ü�������H2��ת����Ϊ40%��| | N2 | H2 | NH3 |
| �� | 1 | 3 | 0 |
| �� | 0.5 | 1.5 | 1 |
| �� | 0 | 0 | 4 |
��ƽ��ʱ���ס��ҡ�����������NH3�����������С˳��Ϊ ��
��4�������Ǻϳ������ԭ�ϣ�д��������������Ӧ����һ����������̬ˮ���Ȼ�ѧ����ʽ ��
 CH3OH(g)��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ����
CH3OH(g)��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ����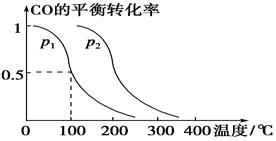
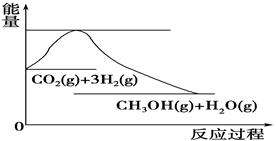
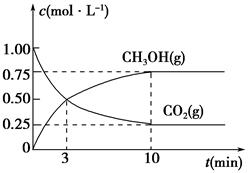
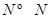 ���ֱ���Ҫ��������436kJ��391KJ��946kJ����N2��H2��Ӧ����1mol NH3(g)���Ȼ�ѧ����ʽ��___________________.
���ֱ���Ҫ��������436kJ��391KJ��946kJ����N2��H2��Ӧ����1mol NH3(g)���Ȼ�ѧ����ʽ��___________________.