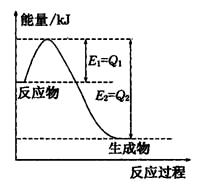
��Ŀ����
�����ŷ�CO2����ɡ�����ЧӦ����Ϊ�˼���úȼ�նԻ�����ɵ���Ⱦ��ú�������Ǹ�Ч���������ú̿����Ҫ;����ú�ۺ����õ�һ��;����ͼ��ʾ��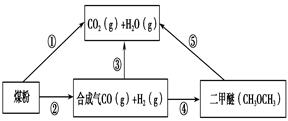
��1����֪��C(s) �� H2O(g) = CO(g)��H2(g) ��H1����131.3 kJ��mol��1
��C(s) �� 2H2O(g) = CO2(g) �� 2H2(g) ��H2����90 kJ��mol��1
��һ����̼��ˮ������Ӧ���ɶ�����̼���������Ȼ�ѧ����ʽ�� ________________________��
��2������ͼԭ���װ�ÿ�����ɹ��̢ݵ�ת������װ��b�缫�ĵ缫��Ӧʽ��_______________________��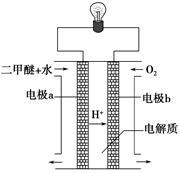
��3����ѹǿΪ0.1 MPa�����£��ݻ�ΪV L���ܱ�������a mol CO��2a mol H2�ڴ��������·�Ӧ���ɼ״���
CO(g)��2H2(g)  CH3OH(g)��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ����
CH3OH(g)��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ����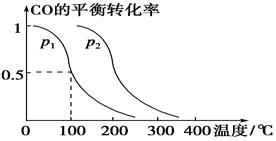
��p1________p2(�����������������)��
���������������������£���������������a mol CO��2a mol H2���ﵽ��ƽ��ʱ��CO��ƽ��ת����________(���������С�����䡱)��
����p1�£�100 ��ʱ��CO(g)��2H2(g)  CH3OH(g)��Ӧ��ƽ�ⳣ��Ϊ________(�ú�a��V�Ĵ���ʽ��ʾ)��
CH3OH(g)��Ӧ��ƽ�ⳣ��Ϊ________(�ú�a��V�Ĵ���ʽ��ʾ)��
��4����ͼ��ʾCO2��H2��Ӧ����CH3OH��H2O�Ĺ���������(��λΪkJ��mol��1)�ı仯��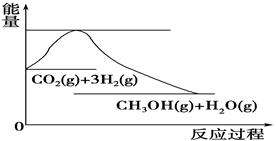
���ڸ÷�Ӧ������˵���У���ȷ����________(����)��
A����H��0����S��0 B����H��0����S��0
C����H��0����S��0 D����H��0����S��0
��5��Ϊ̽����Ӧԭ�����ֽ�������ʵ�飬�����Ϊ1 L���ܱ������У�����1 mol CO2��3 mol H2��һ�������·�����Ӧ��CO2(g)��3H2(g)  CH3OH(g)��H2O(g)�����CO2(g)��CH3OH(g)��Ũ����ʱ��仯��������ͼ��ʾ��
CH3OH(g)��H2O(g)�����CO2(g)��CH3OH(g)��Ũ����ʱ��仯��������ͼ��ʾ��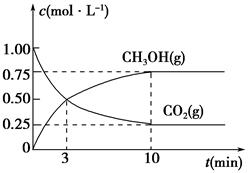
�ٴӷ�Ӧ��ʼ��ƽ�⣬CO2��ƽ����Ӧ����v(CO2)��________��
�����д�ʩ����ʹ��ѧƽ��������Ӧ�����ƶ�����________(����)��
A�������¶� B����CH3OH(g)��ʱҺ���Ƴ�
C��ѡ���Ч���� D���ٳ���1 mol CO2��3 mol H2
��1�� CO(g)��H2O(g)=CO2(g)��H2(g)����H����41.3 kJ��mol��1����2�� O2 +4e�� +2H2O = 4OH���� ��3���٣��� V 2 / a2���������� ��4��C�� ��5����0.075 mol/( L��min). ��BD
���������������1����-�ٿɵã�CO(g)��H2O(g)=CO2(g)��H2(g)����H����41.3 kJ/mol.. ��2����ȼ�ϵ���У�ͨ��ȼ�ϵĵ缫��������ͨ�������ĵ缫��������a�缫�Ǹ�����b�缫��������b�缫�ĵ缫��Ӧʽ��O2 +4e�� +2H2O = 4OH������3�� ����ͼ���Կ��������¶���ͬʱ��ת����P2>P1������ƽ���ƶ�ԭ�����������������������¡�����ѹǿ����ѧƽ�������������С�ķ����ƶ�����������Ӧ�����ƶ�����ʱ��Ӧ���ת������ߡ�����P1<P2. ���������������������£���������������a mol CO��2a mol H2������������ϵ��ѹǿ����ʱ��ѧƽ��������Ӧ�����ƶ����ʴﵽ��ƽ��ʱ��CO��ƽ��ת����������p1�£�100 ��ʱ��CO(g)��2H2(g)  CH3OH(g)��Ӧ��ƽ�ⳣ��ΪK="C" (CH3OH)/ { C(CO)��C2(H2)} ="(" a/2V)��{(a/2V) ��(a/V)}2=" V" 2 / a2.��4��CO2(g)+3H2��g��
CH3OH(g)��Ӧ��ƽ�ⳣ��ΪK="C" (CH3OH)/ { C(CO)��C2(H2)} ="(" a/2V)��{(a/2V) ��(a/V)}2=" V" 2 / a2.��4��CO2(g)+3H2��g�� CH3OH(g)+CO(g).��ͼ�ɿ����÷�Ӧ������Ӧ�Ƿ��ȷ�Ӧ���÷�Ӧ������Ӧ�Ǹ����������С�ķ��ȷ�Ӧ�����ԡ�H<0����S<0��ѡ��Ϊ��C����5���ٴӷ�Ӧ��ʼ��ƽ�⣬CO2��ƽ����Ӧ����v(CO2)=��1-0.25��mol/L��10min="0.075" mol/( L��min). ��A�����¶Ȼ�ѧƽ�������ȷ����ƶ������ڸ÷�Ӧ������Ӧ�Ƿ��ȷ�Ӧ���������¶Ȼ�ѧƽ�����淴Ӧ�����ƶ���B����С�������Ũ�ȣ���ѧƽ��������Ӧ�����ƶ����ʽ�CH3OH(g)��ʱҺ���Ƴ���ʹƽ��������Ӧ�����ƶ���C������ �Ի�ѧƽ����Ӱ�졣D���ﵽƽ��ʱ���ٳ���1 mol CO2��3 mol H2����������ѹǿ����ѧƽ�������������С�ķ�������Ӧ�����ƶ���������ʹ��ѧƽ��������Ӧ�����ƶ��Ĵ�ʩ��BD��
CH3OH(g)+CO(g).��ͼ�ɿ����÷�Ӧ������Ӧ�Ƿ��ȷ�Ӧ���÷�Ӧ������Ӧ�Ǹ����������С�ķ��ȷ�Ӧ�����ԡ�H<0����S<0��ѡ��Ϊ��C����5���ٴӷ�Ӧ��ʼ��ƽ�⣬CO2��ƽ����Ӧ����v(CO2)=��1-0.25��mol/L��10min="0.075" mol/( L��min). ��A�����¶Ȼ�ѧƽ�������ȷ����ƶ������ڸ÷�Ӧ������Ӧ�Ƿ��ȷ�Ӧ���������¶Ȼ�ѧƽ�����淴Ӧ�����ƶ���B����С�������Ũ�ȣ���ѧƽ��������Ӧ�����ƶ����ʽ�CH3OH(g)��ʱҺ���Ƴ���ʹƽ��������Ӧ�����ƶ���C������ �Ի�ѧƽ����Ӱ�졣D���ﵽƽ��ʱ���ٳ���1 mol CO2��3 mol H2����������ѹǿ����ѧƽ�������������С�ķ�������Ӧ�����ƶ���������ʹ��ѧƽ��������Ӧ�����ƶ��Ĵ�ʩ��BD��
���㣺�����Ȼ�ѧ����ʽ����д����ѧƽ�ⳣ���ļ��㼰��������Ի�ѧƽ���Ӱ���֪ʶ��

 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д������Ź㷺����;�������ڻ��ʡ����ᡢ�ϳ���ά�ȹ�ҵ������
��1���������¡��˹��̵������о��������ڳ��¡���ѹ�����������£�N2�ڴ�������������Fe2O3��TiO2��������ˮ������Ӧ�����ɰ�����
�÷�Ӧ�ڹ̶�������ܱ������н��У��й�˵����ȷ����_____________���������ĸ����
A����Ӧ����ƽ��״̬ʱ�� |
B����Ӧ�ﵽƽ��� |
| C����ϵ����ѹǿ���䣬˵����Ӧ�Ѵ�ƽ�� |
| D�����������ܶȱ��ֲ��䣬˵����Ӧ�Ѵ�ƽ�� |
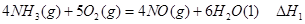 ��
��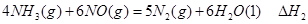 ��
��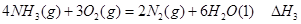 ��
����д������������Ӧ��
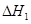 ��
��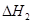 ��
��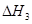 ����֮���ϵ�ı���ʽ��
����֮���ϵ�ı���ʽ��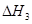 ��_________��
��_________����3����ҵ���������Ҫ��Ӧ�ǣ�
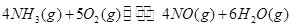
 ��
��
�������¶ȣ���Ӧ��Kֵ��С����Q______���>������<��������0��
������Ӧ��ʼ�����ʵ�����ͬ�����й�ϵͼ�������________������ţ���


�����ݻ��̶����ܱ������з���������Ӧ�������ڲ������ʵ�Ũ�����±���
| ʱ��/Ũ�� |  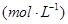 | 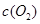 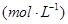 | 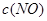 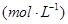 | 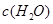 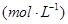 |
| ��ʼ | 4.0 | 5.5 | 0 | 0 |
| ��2min | 3.2 | a | 0.8 | 1.2 |
| ��4min | 2.0 | 3.0 | 2.0 | 3.0 |
| ��6min | 2.0 | 3.0 | 2.0 | 3.0 |
��Ӧ�ڵ�2 min����4 minʱ��O2��ƽ����Ӧ����Ϊ________��
��Ӧ�ڵ�2 minʱ�ı����������ı������������______________________________��
�������£���Ӧ��ƽ�ⳣ��K��________��
����β���ﺬ�е�NO������������ȼ��ȼ�յĸ�����������������Ӧ���£�
N2(g)��O2(g)  2NO(g) ��H����֪�÷�Ӧ�� T ��ʱ��ƽ�ⳣ��K��9.0��
2NO(g) ��H����֪�÷�Ӧ�� T ��ʱ��ƽ�ⳣ��K��9.0��
��ش�
��1����֪��N2(g)+2O2(g)  2NO2(g) ��H1 2NO2(g)
2NO2(g) ��H1 2NO2(g)  O2+2NO(g) ��H2 ��H= ���ú���H1����H2�ı���ʽ��ʾ����
O2+2NO(g) ��H2 ��H= ���ú���H1����H2�ı���ʽ��ʾ����
��2��ij�¶��£���2 L���ܱ������г���N2��O2��1 mol��5���Ӻ�O2�����ʵ���Ϊ0.5 mol����NO�ķ�Ӧ���� ��
��3���ٶ��÷�Ӧ���ں��������½��У��������жϸ÷�Ӧ�Ѵﵽƽ�����________��
| A������1 mol N2ͬʱ����1 mol O2 |
| B����������ܶȲ��� |
| C���������ƽ����Է����������� |
| D��2v��(N2)��v��(NO) |
 2NO(g)�ġ�K-T������c(NO)-t��ͼ����ͼA������֪�÷�ӦΪ ��Ӧ������ȡ����ȡ�������ͼB��֪����a��Ӧ��������ȣ�b�ı������������ ��
2NO(g)�ġ�K-T������c(NO)-t��ͼ����ͼA������֪�÷�ӦΪ ��Ӧ������ȡ����ȡ�������ͼB��֪����a��Ӧ��������ȣ�b�ı������������ ��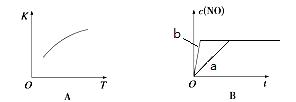
��5��T ��ʱ��ijʱ�̲��������N2��O2��NO��Ũ�ȷֱ�Ϊ0.20 mol��L��1��0.20mol��L��1��0.50mol��L��1����ʱ��ӦN2(g)��O2(g)
 2NO(g)________________(����ڻ�ѧƽ��״̬������������Ӧ������С������淴Ӧ������С�)��ƽ��ʱ��N2�ڻ�����������ٷ���Ϊ���٣����ڴ����д�����������̣��������2λ��Ч���֣�
2NO(g)________________(����ڻ�ѧƽ��״̬������������Ӧ������С������淴Ӧ������С�)��ƽ��ʱ��N2�ڻ�����������ٷ���Ϊ���٣����ڴ����д�����������̣��������2λ��Ч���֣�  ����ȡ�״����䷴ӦΪ��CO2+3H2
����ȡ�״����䷴ӦΪ��CO2+3H2 CH3OH+H2O ���³�ѹ����֪���з�Ӧ�������仯��ͼʾ��
CH3OH+H2O ���³�ѹ����֪���з�Ӧ�������仯��ͼʾ��

 CH3OCH3��H2O
CH3OCH3��H2O CH3OCH3(g)��CO2(g) ��H����247kJ/mol
CH3OCH3(g)��CO2(g) ��H����247kJ/mol


 ��Һ���ܡ����������е�
��Һ���ܡ����������е�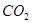 ��
��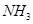 Ϊԭ�Ͽɺϳɻ�������[
Ϊԭ�Ͽɺϳɻ�������[ ]����֪��
]����֪��
 ��
��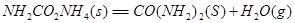
 ��
��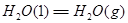
 ��
�� ���ڴ���������CO��
���ڴ���������CO��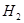 ��Ӧ���ɼ״���
��Ӧ���ɼ״��� ij�ݻ��ɱ���ܱ������г���10molCO��20mol
ij�ݻ��ɱ���ܱ������г���10molCO��20mol
 _______VL��������ڡ�����С�ڡ����ڡ���
_______VL��������ڡ�����С�ڡ����ڡ���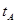 _______
_______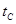 ���>������<����=����
���>������<����=����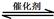 2CO2(g)+ N2(g)����H��0
2CO2(g)+ N2(g)����H��0
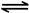 N2O4(g) ��H����56.9 kJ/mol
N2O4(g) ��H����56.9 kJ/mol 
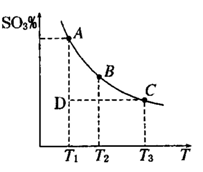
 2SO3��g���������ϵ��SO3 �İٷֺ������¶ȵĹ�ϵ����ͼ��ʾ���������κ�һ�㶼��ʾƽ��״̬��������ͼʾ�ش��������⣬
2SO3��g���������ϵ��SO3 �İٷֺ������¶ȵĹ�ϵ����ͼ��ʾ���������κ�һ�㶼��ʾƽ��״̬��������ͼʾ�ش��������⣬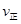
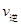 ���>����<����=����
���>����<����=����