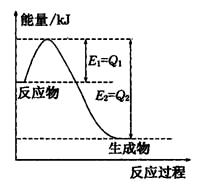
��Ŀ����
(1)CH4(g )+2O2(g )=CO2(g )+2H2O(g ) ��H=-802.3kJ/mol
���Ȼ�ѧ��Ӧ����ʽ��������_____________________________________��
(2)��֪2g�Ҵ���ȫȼ������Һ̬ˮ�ų�Q kJ��������д����ʾ�Ҵ�ȼ���ȵ��Ȼ�ѧ��
��ʽ��____________________________________________________________.
(3)��֪��1mol H-H����1mol N-H����1mol  ���ֱ���Ҫ��������436kJ��391KJ��946kJ����N2��H2��Ӧ����1mol NH3(g)���Ȼ�ѧ����ʽ��___________________.
���ֱ���Ҫ��������436kJ��391KJ��946kJ����N2��H2��Ӧ����1mol NH3(g)���Ȼ�ѧ����ʽ��___________________.
(4)���ݸ�˹���ɿ��Զ�ijЩ����ͨ��ʵ��ֱ�Ӳⶨ�Ļ�ѧ��Ӧ���ʱ�������㡣
��֪��C(ʯī��s)+O2(g)=CO2(g) ��H=-393.5kJ/mol ��
2H2(g)+O2(g)=2H2O(l) ��H=-571.6kJ/mol ��
2C2H2(g)+5O2(g)=4CO2(g)+2H2O(l) ��H=-2599kJ/mol ��
���ݸ�˹���ɣ�����298Kʱ��C��ʯī��s����H2(g)����1mol C2H2(g)��Ӧ���ʱ䣺
____________________________.
��1��1mol���������2mol��������ȫ��Ӧ����1mol������̼��2molˮ����ʱ�ų�802.3KJ����������2��C2H5OH(l)+3O2(g)=2CO2(g)+3H2O(l) ; ��H=-23QKJ�Mmol (3)( 1�M2) N2(g)+( 3�M2) H2(g)=NH3(g) ; ��H=-46KJ�Mmol (4)+226.7KJ�Mmol��
���������������1�����Ȼ�ѧ��Ӧ����ʽ��������1mol���������2mol��������ȫ��Ӧ����1mol������̼��2molˮ����ʱ�ų�����802.3KJ����2���Ҵ�����Է���������46.1mol�Ҵ�������Ϊ46g����Ϊ2g�Ҵ�ȼ������Һ̬ˮ����Q kJ������46g�Ҵ�ȼ������Һ̬ˮʱ�ų�������Ϊ23 Q kJ��������ȼ���ȵ��Ȼ�ѧ����ʽΪ��C2H5OH(l)+3O2(g)=2CO2(g)+3H2O(l) ; ��H=-23QKJ�Mmol����Ӧ���Ƕ��ѻ�ѧ�����յ���������ѧ�����ͷŵ����������N2��H2��Ӧ����1mol NH3(g)�ķ�Ӧ��Ϊ( 1�M2)��946+( 3�M2) ��436-3��391=-46.�ʸ÷�Ӧ���Ȼ�ѧ����ʽ��( 1�M2) N2(g)+( 3�M2) H2(g)=NH3(g) ; ��H="-46KJ�Mmol" (4) �١�2+��1�M2 ������-��1�M2 �����۵æ�H=2��(-393.5)+��1�M2 ����(-571.6)+��1�M2 ����2599=+226.7KJ�Mmol.���÷�Ӧ���Ȼ�ѧ����ʽΪ��2C(ʯī��s)+H2(g)=C2H2(g ) ; ��H=+226.7KJ�Mmol.
���㣺�����˹����Ӧ�á��Ȼ�ѧ����ʽ����д���塢���뻯ѧ���Ĺ�ϵ�ȵ�֪ʶ��

 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д���������Ԫ���γɵij���������H2O��H2O2����һ�������¾��ɷֽ⡣
��1����֪��
| ��ѧ�� | �Ͽ�1mol��ѧ�������������kJ�� |
| H��H | 436 |
| O��H | 463 |
| O=O | 498 |
��H2O��g���ֽ���Ȼ�ѧ����ʽ�� ��
��11.2 L����״������H2��ȫȼ�գ�������̬ˮ���ų� kJ��������
��2��ijͬѧ��H2O2�ֽ�Ϊ����̽��Ũ������Һ����ԶԷ�Ӧ���ʵ�Ӱ�졣�����£����������ʾ�ķ������ʵ�顣
| ʵ���� | ��Ӧ�� | ���� | |
| a | 50 mL 5% H2O2��Һ | | 1 mL 0.1 mol��L-1 FeCl3��Һ |
| b | 50 mL 5% H2O2��Һ | ����Ũ���� | 1 mL 0.1 mol��L-1 FeCl3��Һ |
| c | 50 mL 5% H2O2��Һ | ����ŨNaOH��Һ | 1 mL 0.1 mol��L-1 FeCl3��Һ |
| d | 50 mL 5% H2O2��Һ | | MnO2 |
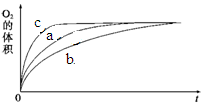
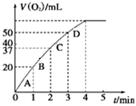
ͼ1 ͼ2
�ɸ�ͼ�ܹ��ó���ʵ�������_________��
�ڲ��ʵ��d�ڱ�״���·ų������������ʱ��仯�Ĺ�ϵ��ͼ2��ʾ�����ͷ�Ӧ���ʱ仯��ԭ�� ��
ʵʩ�Լ�����Դ�˷Ѻͽ��ͷ����ŷ�Ϊ�������ݵĽ��ܼ������ߣ���Ӧ��ȫ���������⡢������Դ��Լ�͡������Ѻ������ı�Ȼѡ������ҵ�ķ�չ������Ϲ��ҽ��ܼ��ŵ�����Ҫ����������ѧ֪ʶ������������⣺
����֪ij��Ӧ��ƽ�����ʽΪ��
������Ӧ�Ļ�ѧ��Ӧ����ʽΪ��
������ˮú���ϳɶ����ѵ�������Ӧ���£�
��2H2��g��+CO��g�� CH3OH��g������H=��90.8kJ��mol
CH3OH��g������H=��90.8kJ��mol
��2CH3OH��g�� CH3OCH3��g��+H2O��g������H=��23.5kJ��mol
CH3OCH3��g��+H2O��g������H=��23.5kJ��mol
��CO��g��+H2O��g�� CO2��g��+H2��g������H=��41.3kJ��mol
CO2��g��+H2��g������H=��41.3kJ��mol
�ܷ�Ӧ��3H2��g��+3CO��g�� CH3OCH3��g��+CO2��g���Ħ�H=__________
CH3OCH3��g��+CO2��g���Ħ�H=__________
(3)ú����ͨ��ͨ���о���ͬ�¶���ƽ�ⳣ���Խ������ʵ�����⡣��֪�������һ����̼��ˮ�������뷴Ӧ��ʱ���ᷢ�����·�Ӧ��CO��g��+H2O��g�� H2��g��+CO2��g�����÷�Ӧƽ�ⳣ�����¶ȵı仯���±���ʾ��
H2��g��+CO2��g�����÷�Ӧƽ�ⳣ�����¶ȵı仯���±���ʾ��
| �¶�/�� | 400 | 500 | 800 |
| ƽ�ⳣ��K | 9.94 | 9 | 1 |
�÷�Ӧ������Ӧ�����Ƿ�Ӧ������ȡ����ȡ���������500��ʱ���У�����ʼʱCO��H2O����ʼŨ�Ⱦ�Ϊ0.020mol/L���ڸ������£�CO��ƽ��ת����Ϊ����
(4)�Ӱ����������������ᣬ�˹������漰���������NO��NO2��N2O4�ȡ�
�Է�Ӧ:N2O4��g��
 2NO2��g����H>0�����¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ���ǡ�
2NO2��g����H>0�����¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ���ǡ�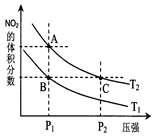
A��A��C����ķ�Ӧ���ʣ�A��C
B��A��C�����������ɫ��A�Cdz
C��B��C����������ƽ����Է���������B��C
D����״̬B��״̬A�������ü��ȵķ���
E��A��C����Ļ�ѧƽ�ⳣ����A��C
(5)CO2(g)+3H2(g)
 CH3OH(g)+H2O(g)��H=��49.0kJ��mol��1�������¶ȡ��ݻ���ͬ��3���ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ���й��������¡�����˵����ȷ����
CH3OH(g)+H2O(g)��H=��49.0kJ��mol��1�������¶ȡ��ݻ���ͬ��3���ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ���й��������¡�����˵����ȷ����
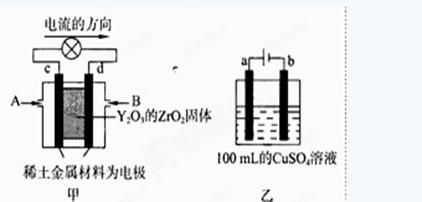
��������;�㷺����Ҫ��������Ӳ�ʻ����µĺϽ��Լ����ݵĵ�˿�������£����ܱ���������H2��ԭWO3�ɵõ������٣����ܷ�ӦΪ��
WO3 (s) + 3H2 (g) W (s) + 3H2O (g)
W (s) + 3H2O (g)
��ش��������⣺
��������Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ___________________________��
��ij�¶��·�Ӧ��ƽ��ʱ��H2��ˮ�����������Ϊ2:3����H2��ƽ��ת����Ϊ_____________________�����¶ȵ����ߣ�H2��ˮ����������ȼ�С����÷�ӦΪ��Ӧ_____________________������ȡ����ȡ�����
�������ܷ�Ӧ���̴��·�Ϊ�����Σ�������Ҫ�ɷ����¶ȵĹ�ϵ���±���ʾ��
| �¶� | 25�� ~ 550�� ~ 600�� ~ 700�� |
| ��Ҫ�ɷ� | WO3 W2O5 WO2 W |
��һ�η�Ӧ�Ļ�ѧ����ʽΪ___________________________��580��ʱ���������ʵ���Ҫ�ɷ�Ϊ________������WO3��ȫת��ΪW��������������H2���ʵ���֮��Ϊ____________________________________��
�� ��֪���¶ȹ���ʱ��WO2 (s)ת��ΪWO2 (g)��
WO2 (s) + 2H2 (g)
 W (s) + 2H2O (g)����H �� +66.0 kJ��mol��1
W (s) + 2H2O (g)����H �� +66.0 kJ��mol��1 WO2 (g) + 2H2(g)
 W (s) + 2H2O (g)����H �� ��137.9 kJ��mol��1
W (s) + 2H2O (g)����H �� ��137.9 kJ��mol��1 ��WO2 (s)
 WO2 (g) �Ħ�H �� ______________________��
WO2 (g) �Ħ�H �� ______________________������˿�ƹ��е�W��ʹ�ù����л����ӷ���ʹ��˿��ϸ������I2���ӳ��ƹܵ�ʹ���������乤��ԭ��Ϊ��W (s) +2I2 (g)
 WI4 (g)������˵����ȷ����____________��
WI4 (g)������˵����ȷ����____________��a���ƹ��ڵ�I2��ѭ��ʹ��
b��WI4�ڵ�˿�Ϸֽ⣬������W�ֳ����ڵ�˿��
c��WI4�ڵƹܱ��Ϸֽ⣬ʹ�ƹܵ������ӳ�
d���¶�����ʱ��WI4�ķֽ����ʼӿ죬W��I2�Ļ������ʼ���
�ϳɰ���ҵ�Թ���������Ҫ���壬�������ᡢ�ϳ���ά�Լ�Ⱦ�ϵ�
��1����֪ijЩ��ѧ���ļ����������±���
| ��ѧ�� | N��N | H��H | N��H |
| ����kJ��mol��1 | 946 | 436 | 390 |
�ϳɰ����Ȼ�ѧ��Ӧ����ʽΪ ��
��2�������£���NaCl�뱥�Ͱ�ˮ�Ļ����Һ��ͨ�����CO2����NaHCO3�����������÷�Ӧ�Ļ�ѧ����ʽΪ ��������Һ�����ӵĵ���غ�ʽ�� ������NaCl�뱥��CO2�Ļ����Һ��ͨ�백������û��NaHCO3����������ԭ���� ��
��3��������ˮ(��NH3��NaOH��Na2SO4)�����ŷŻ����ˮ�帻Ӫ��������ͼͨ��ֱ�ӵ绯ѧ��������Ч��ȥij�������������������ӵ���������Ϊ �����a��������b���������������У�b������pH �������С�����䡱����������Ӧ����ʽΪ ��
 ����ȡ�״����䷴ӦΪ��CO2+3H2
����ȡ�״����䷴ӦΪ��CO2+3H2 CH3OH+H2O ���³�ѹ����֪���з�Ӧ�������仯��ͼʾ��
CH3OH+H2O ���³�ѹ����֪���з�Ӧ�������仯��ͼʾ��

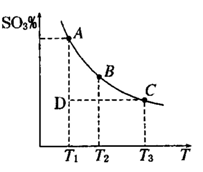
 2SO3��g���������ϵ��SO3 �İٷֺ������¶ȵĹ�ϵ����ͼ��ʾ���������κ�һ�㶼��ʾƽ��״̬��������ͼʾ�ش��������⣬
2SO3��g���������ϵ��SO3 �İٷֺ������¶ȵĹ�ϵ����ͼ��ʾ���������κ�һ�㶼��ʾƽ��״̬��������ͼʾ�ش��������⣬
 ���>����<����=����
���>����<����=����