��Ŀ����
����Ŀ����1����Ҫ��д������ʽ��
��HNO3 (���뷽��ʽ��_____
��Fe2(SO4)3 (���뷽��ʽ��_____
�������ƺ��Ȼ�����Һ��Ӧ�����ӷ���ʽ��____
��������̼ͨ����������������Һ�����ӷ���ʽ��____________
��2����0.4molCH4����������ԭ������__g HCl����������ԭ�����������HC1�����ڱ�״���µ����Ϊ________ L��
�������ʵ���O2�ͳ���(O3)��������֮��Ϊ_______����O2��O3������ȣ�����ԭ����֮��Ϊ__��
��3������Ϊ��ѧ��ѧ�г����ļ����������ٶ�����̼������KC1��NaHSO4�����ͭ��ϡ�������ʯ��ˮ���������ڵ���ʵ���_____�����ڷǵ���ʵ���____(���ţ���
��4����������(Na2FeO4)(��Ϊ+6�ۣ���һ�����͵ľ�ˮ��������ͨ��������Ӧ��ȡ�� 2Fe (OH) 3 + 4NaOH + 3NaC1O = 2Na2FeO4 + 3NaCl + 5H2O
���÷�Ӧ����������_________(�û�ѧʽ��ʾ����ͬ)��______Ԫ�ر���������ԭ��Ϊ__________ ��
���õ������ڷ���ʽ�б������ת�Ƶ������_______________
������Ӧ����lmolNaFeO4���ɣ�ת�Ƶĵ�����_________ mol��
��ʵ����������250mL0.1 molL-1NaOH��Һ�����ձ�������������Ͳ����ͷ�ιܣ�����Ҫ�õ��IJ�������Ϊ__________�����в������Ƶ���ҺŨ��ƫ�͵���__________��
A.����NaOHʱ����NaOH����ֽ�ϳ���
B.����ǰ������ƿ������������ˮ
C.����ʱ��NaOHδ��ȴֱ�Ӷ���
D.������ƿ��ת����Һʱ������Һ����������ƿ����
E.����ʱ���ӿ̶���
���𰸡�HNO3=H++NO3- Fe2(SO4)3=2Fe3++3SO42�� Ba2++ SO42-= BaSO4�� CO2+2OHһ=CO32һ+ H2O 36.5 22.4 2:3 1:1 �ڢ� �� NaClO Fe NaCl ![]() 3 250mL����ƿ AD
3 250mL����ƿ AD
��������
��1��ע��ǿ�������ȫ���룬������ʲ��ֵ��롣���ӷ���ʽ��Ҫע��������ʡ������������ﲻ�ܲ�д��
��2�����������ʵ���������µ��������������Ŀ֮��Ĺ�ϵ������n= ![]() =
= ![]() =
= ![]() ���л��㡣
���л��㡣
��3�����������ˮ��Һ������״̬���ܵ���Ļ��������ǵ��������ˮ��Һ������״̬�¶����ܵ���Ļ����
��4��2Fe (OH) 3 + 4NaOH + 3NaC1O = 2Na2FeO4 + 3NaCl + 5H2O���÷�Ӧ��������NaC1O����ԭ��ΪFe (OH) 3��ÿ2mol Fe (OH) 3��������ת��6mol���ӣ�3mol NaC1O����ԭ��
������Һʱ����Һ�����ʵ���Ũ��c=![]() ���ݴ��ж�ʵ�������Ũ�ȱ仯��
���ݴ��ж�ʵ�������Ũ�ȱ仯��
��1��������Ϊǿ����ʣ�������ȫ���������뷽��ʽΪ��HNO3=H++NO3-��
��������Ϊǿ����ʣ�������ȫ���������뷽��ʽΪ��Fe2(SO4)3=2Fe3++3SO42��-��
�������ƺ��Ȼ�����Һ��Ӧ�������ᱵ���Ȼ��ƣ����ᱵΪ�������ܲ�д�����ӷ���ʽΪ��Ba2++ SO42-= BaSO4����
�ܶ�����̼����������������Һ��Ӧ������̼���ƺ�ˮ�����ӷ���ʽΪ��CO2+2OHһ=CO32һ+ H2O��
��2����0.4molCH4�����к���2molԭ�ӣ�1mol HCl�����к���2molԭ�ӣ�1mol HCl���ӵ�����Ϊm=n��M=1mol��36.5g/mol=36.5g��1mol HC1�����ڱ�״���µ����V= n��Vm=1mol��22.4L/mol=22.4L����Ϊ��36.5 ��22.4��
�ڵ����ʵ���O2�ͳ���(O3)��������֮��Ϊ��n��32g/mol������n��48g/mol��=2��3�������ͳ���������ԭ�ӹ��ɣ���O2��O3������ȣ�����ԭ������ȣ���ԭ�Ӹ���֮��Ϊ1:1����Ϊ��2:3��1:1��
��3����ˮ��Һ������״̬���ܵ���Ļ������ǵ���ʣ����ڵ���ʵ�����������KC1��NaHSO4���壻��ˮ��Һ������״̬�¶����ܵ���Ļ������Ƿǵ���ʣ����ڷǵ���ʵ������ٶ�����̼����Ϊ���ڢۣ��١�
��4��������������ԭ�����ϼ۽��ͣ��÷�Ӧ��������ΪNaC1O���÷�Ӧ����������NaClO ����ԭ��ΪFe (OH) 3����������Ԫ��ΪFeԪ�أ���ԭ����ΪNaCl����Ϊ��NaC1O��Fe��NaCl��
���õ������ڷ���ʽ�б������ת�Ƶ������![]()
��ÿ2mol Fe (OH) 3��������ת��6mol����������2mol Na2FeO4������Ӧ����lmolNaFeO4���ɣ�ת�Ƶĵ�����3mol����Ϊ��3��
��ʵ����������250mL0.1 molL-1NaOH��Һ�����ձ�������������Ͳ����ͷ�ιܣ�����Ҫ�õ��IJ�������Ϊ250mL����ƿ����Ϊ��250mL����ƿ��
������Һʱ����Һ�����ʵ���Ũ��c=![]() ��A.���������׳��⣬��ֽ����������������������ֽ�ϣ��ܽ�����������������٣�A����������B.����ǰ������ƿ������������ˮ����������Һ��Ũ����Ӱ�죬B�����ϣ� C.��Һ��ȴ�������ٶ��ݣ�δ��ȴֱ�Ӷ��ݣ�����ȴ�����£���Һ�����С����ҺŨ�ȱ��C�����ϣ� D.������ƿ��ת����Һʱ������Һ����������ƿ���棬���ʼ��٣���ҺŨ������D�������⣻ E.����ʱ���ӿ̶��ߣ���Һ���ƫС����ҺŨ��ƫ�ߣ�E�����ϡ���Ϊ��AD��
��A.���������׳��⣬��ֽ����������������������ֽ�ϣ��ܽ�����������������٣�A����������B.����ǰ������ƿ������������ˮ����������Һ��Ũ����Ӱ�죬B�����ϣ� C.��Һ��ȴ�������ٶ��ݣ�δ��ȴֱ�Ӷ��ݣ�����ȴ�����£���Һ�����С����ҺŨ�ȱ��C�����ϣ� D.������ƿ��ת����Һʱ������Һ����������ƿ���棬���ʼ��٣���ҺŨ������D�������⣻ E.����ʱ���ӿ̶��ߣ���Һ���ƫС����ҺŨ��ƫ�ߣ�E�����ϡ���Ϊ��AD��

 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�����Ŀ����ͼ����ѧ��ѧ�г������ݺ���ķ�����ᴿװ�ã������װ�ûش����⣺
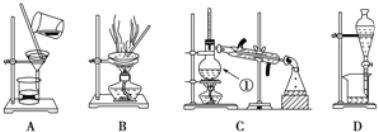
��1����װ��A��װ��B�ж��õ���������װ��A�в�������������_____��װ��B�в�������������_________��
��2��װ��C������������_____����װ��������ˮ����������_____��װ��D�еķ�Һ©����ʹ��֮ǰӦ��_____���ڷ�ҺʱΪʹҺ��˳�����£�Ӧ���еľ��������_______��
��3��ij�����ƹ����л����������������ʣ������һʵ�鷽�����ȳ�ȥ���ʣ��������������Һ��
ʵ�鷽�����Ƚ�������������ˮ�����Һ��ѡ����ʵ��Լ��Ͳ�����ɱ����и���ʵ�顣
ѡ���Լ� | �� | Na2CO3��Һ | �� |
ʵ����� | �� | �� | ���� |
��������Լ��ٿ�����_____���ѧʽ����֤����Һ��SO42-�Ѿ������ķ�����______������Na2CO3��Һ��Ŀ����_____����������Լ��ܿ�����_____���ѧʽ����
��4���������Ǹ�Ч�Ŀ�ű��ҩ��Ϊ��ɫ��״���壬�����ڱ�ͪ���ȷºͱ��У��ڼ״����Ҵ������ѡ�ʯ�����п��ܽ⣬��ˮ�м������ܣ��۵�Ϊ156-157�棬���ȶ��Բ��֪�����ѷе�Ϊ35�档��ȡ�����ص���Ҫ����Ϊ��

��Ҫ��ʵ����ģ���������գ�����Iѡ���װ����_____������ţ����������������_____��ѡ���װ����_____������ţ���Ϊ��ֹ���У���Ʒ����Ҫ����_____������������Ҫ���̿�����_____������ĸ����
A.��ˮ�ܽ⣬����Ũ������ȴ�ᾧ������
B.��95%���Ҵ���Ũ�����ᾧ������
C.�������ѽ�����ȡ��Һ
����Ŀ��ij�����ĵ�������к���ͭ�����Ƚ��������Ϊʵ����Դ�Ļ������ò���Ч��ֹ������Ⱦ��������¹������̣�

������ | Fe(OH)3 | Fe(OH)2 | Cu(OH)2 |
��ʼ������pH | 2.3 | 7.6 | 4.4 |
��ȫ������pH | 3.2 | 9.7 | 6.4 |
��1����������H2O2��Ŀ����________________����pH�������˷�ΧΪ________________��
��2����pH�����м�����Լ������___________��
A. NaOH B. CuO C.NH3��H2O D.HCl
��3�����CuSO4��Һ��ԭ����_________����CuSO4��Һ�м���һ������NaCl��Na2SO3���������ɰ�ɫ��CuCl������д���÷�Ӧ�Ļ�ѧ����ʽ_________��
��4�����˺�ij�������ͨ��������һЩ�������ӣ�Ϊ�õ����������Ҫ����ϴ�ӣ�ȷ������ϴ�Ӹɾ��IJ�����������_________��
��5����ȡ���Ʊ���CuCl��Ʒ0.2500g����һ������0.5mol��L-1FeCl3��Һ�У�����Ʒ��ȫ�ܽ��ˮ20mL����0.1000 mol��L-1��Ce(SO4)2��Һ�ζ��������յ�ʱ����Ce(SO4)2��Һ25.00mL���йصĻ�ѧ��ӦΪFe3++CuCl=Fe2++Cu2++Cl-��Ce4++Fe2+=Fe3++Ce3+�������CuCl��Ʒ����������__________��



