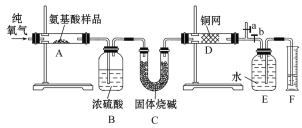
��Ŀ����
����Ŀ�����������գ�
(һ)���� 8 �����ʻ����ӣ�
��14N �� 14C ��16O �� 18O �������(NH4CNO)������[CO(NH2)2] ������ϩ(C60)�ͽ��ʯ ��CH3CH2CH2CH3 �� CH(CH3)3 ��CH3CH2CH3 �� CH3(CH2)2CH3 �� ![]() ��
�� ![]() ��
��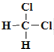 ��
��![]()
(1)��Ϊͬ�����������__________(���ţ���ͬ)��
(2)��Ϊͬλ�ص���________________��
(3)��Ϊͬϵ�����___________��
(4)��Ϊͬ���칹�����________________��
(��)�����м��־��壺
A��ˮ�� B�������� C������ D�����ʯ E������� F���ɱ� G�������� H����������
(1)����ԭ�Ӿ���Ļ�������__________________(����ţ���ͬ)��
(2)���й��ۼ������Ӿ�����________________________��
(3)�����ڻ�ʱ��Ҫ�˷����ۼ��ľ�����_________________________��
(4)д���������ʵĵ���ʽ����������_____________��������̼________________��
(��)ij�����Ľṹ��ʽΪ��
(1)��ϵͳ����������������__________________________��
(2)������������ϩ������õ��ģ���ԭϩ���Ľṹ������ ________��(�����������칹����ͬ)��
(3)������������Ȳ������õ��ģ���ԭ�����Ľṹ��_________________�֣�
(4)�������ڹ�����������������Ӧ�����ɵ�һ�����������____________�֡�
(��)����ƽ�����ʢ��ǿ��ԭ��Һ̬��(N2H4)��ǿ������Һ̬˫��ˮ�� ���� 0.4mol Һ̬�º�0.8mol H2O2 ��Ϸ�Ӧ�����ɵ�����ˮ�������ų� 256.7KJ ������(�൱�� 25�桢101 kPa �²�õ�����)��
�ٷ�Ӧ���Ȼ�ѧ����ʽΪ________________________________��
����֪ H2O(1)=H2O(g) ��H=+44kJ/mol��
�� 16g Һ̬����Һ̬˫��ˮ��Ӧ����Һ̬ˮʱ�ų���������_________________kJ��
�۴˷�Ӧ���ڻ���ƽ������ͷŴ����ȺͿ��ٲ������������⣬����һ���ܴ���ŵ���____________��
���𰸡��� �� �� �ۢ� A H AD ![]()
![]() 2,3-�������� 5 1 6 N2H4(g)+2H2O2(l)=N2(g)+4H2O(g) ��H=-641.75kJ/mol 408.875 ���ﻷ������Ⱦ
2,3-�������� 5 1 6 N2H4(g)+2H2O2(l)=N2(g)+4H2O(g) ��H=-641.75kJ/mol 408.875 ���ﻷ������Ⱦ
��������
��һ��ͬһ��Ԫ����ɵIJ�ͬ���ʻ�Ϊͬ�������壻ͬһ��Ԫ�صIJ�ͬ���ػ�Ϊͬλ�أ�����ʽ��ͬ���ṹ��ͬ�Ļ����ﻥΪͬ���칹�壻�ṹ���ƣ�������������һ�������ɸ�CH2���л������ﻥΪͬϵ����������������жϣ�
��14N��14C���ֲ�ͬ��Ԫ�أ���16O��18O������������ͬ����������ͬ��ͬһ��Ԫ�صIJ�ͬ���أ���ͬλ�أ��������(NH4CNO)������[CO(NH2)2]�����߷���ʽ��ͬ���ṹ��ͬ����ͬ���칹�壻������ϩ(C60)�ͽ��ʯ�����߾�����̼Ԫ�ع��ɵIJ�ͬ���ʣ�����ͬ�������壻��CH3CH2CH2CH3��CH(CH3)3�����߷���ʽ��ͬ���ṹ��ͬ����ͬ���칹�壻��CH3CH2CH3��CH3(CH2)2CH3���������������ṹ���ƣ�������������һ��CH2���л������ﻥΪͬϵ��� ![]() ��
�� ![]() �����߽ṹ��ͬ������ʽ��ͬ����ͬһ�����ʣ���
�����߽ṹ��ͬ������ʽ��ͬ����ͬһ�����ʣ���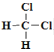 ��
��![]() �����߽ṹ��ͬ������ʽ��ͬ����ͬһ�����ʣ�
�����߽ṹ��ͬ������ʽ��ͬ����ͬһ�����ʣ�
��Ϊ:��Ϊͬ����������Ǣܣ���Ϊͬλ�ص��Ǣڣ���Ϊͬϵ����Ǣޣ���Ϊͬ���칹����Ǣۢݡ�
������ˮ���ǹ�ԭ������ԭ��֮��ͨ�����ۼ�����γɵĿռ���״�ṹ�ľ�����ԭ�Ӿ��壬��O-Siԭ�Ӽ��γɼ��Լ������ڻ�����������Ǵ�����Ӽ�ͨ�����Ӽ�����������γɵľ���Ϊ���Ӿ��壻������ͨ�����Ӽ�����������γɵľ���Ϊ���Ӿ��壻���ʯ��̼ԭ��֮��ͨ�����ۼ�����γɵĿռ���״�ṹ�ľ�����ԭ�Ӿ��壬��C-Cԭ�Ӽ��γɷǼ��Լ������ڵ��ʣ�������ǵ�ԭ�ӹ��ɵģ���ѧ����ͨ�����Ӽ�����������γɵķ��Ӿ��壻�ɱ���CO2���Ӽ�ͨ�����Ӽ�����������γɵľ���Ϊ���Ӿ��壻����������������������γ����Ӽ����������Ӿ��壻������������������������γ����Ӽ�����ԭ������ԭ���γɹ��ۼ������������м������Ӽ����й��ۼ����������Ӿ��壻���Ϸ�����֪��
(1)����ԭ�Ӿ���Ļ�������ˮ����(2)���й��ۼ������Ӿ����ǹ������ƣ�(3)�����ڻ�ʱ��Ҫ�˷����ۼ��ľ�����ԭ�Ӿ���ˮ���ͽ��ʯ�����Ӿ����ڻ�ֻ��˷����Ӽ������������Ӿ����ڻ��˷����Ӽ���(4)������������������������γ����Ӽ�����ԭ������ԭ���γɹ��ۼ��������ʽΪ![]() ��CO2��̼ԭ����ÿ����ԭ���γ����Թ��õ��Ӷԣ������ʽΪ
��CO2��̼ԭ����ÿ����ԭ���γ����Թ��õ��Ӷԣ������ʽΪ![]() ��
��
����A��H��AD��![]() ��
��![]() ��
��
��������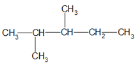 ������
������
(1)���л����̼����������Ϊ5��̼ԭ�ӣ���ţ�2��λ��һ������3��λ��һ������ϵͳ����Ϊ2,3-�������飻
��Ϊ2��3-�������顣
(2)������������ϩ������õ��ģ���ԭϩ���Ľṹ�� ��
��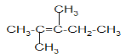 ��
��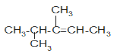 ��
�� ��
�� ����5�֣�
����5�֣�
��Ϊ:5��
(3)��������ΪȲ�������Ƶã��ڸ�̼������̼̼������ֻ��һ�ַ�ʽ�����Դ�Ȳ���Ľṹ��ʽΪ��![]() ��
��
��Ϊ:1��
(4)��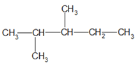 �������к���6�ֲ�ͬ����ԭ�ӣ����Ը���������6��һ�ȴ��飻
�������к���6�ֲ�ͬ����ԭ�ӣ����Ը���������6��һ�ȴ��飻
��Ϊ:6��
���ģ���0.4molҺ̬�·ų�256.7kJ����������1molҺ̬�·ų�������Ϊ![]() =641.75kJ�����Է�Ӧ���Ȼ�ѧ����ʽΪ��N2H4(l)+2H2O2(l)=N2(g)+4H2O(g)��H=-641.75kJ/mol����Ϊ��N2H4(g)+2H2O2(l)=N2(g)+4H2O(g)��H=-641.75kJ/mol��
=641.75kJ�����Է�Ӧ���Ȼ�ѧ����ʽΪ��N2H4(l)+2H2O2(l)=N2(g)+4H2O(g)��H=-641.75kJ/mol����Ϊ��N2H4(g)+2H2O2(l)=N2(g)+4H2O(g)��H=-641.75kJ/mol��
���ɢ�N2H4(l)+2H2O2(l)�TN2(g)+4H2O(g)��H=-641.75kJ/mol����H2O(l)=H2O(g)��H=+44kJ/mol�����ݸ�˹���ɢ�-����4�õ�N2H4(l)+2H2O2(l)�TN2(g)+4H2O(l)��H=-817.75kJ/mol����16gҺ̬�¼�![]() =0.5mol����Һ̬˫��ˮ��Ӧ����Һ̬ˮʱ�ų�������0.5mol��817.75kJ/mol=408.875kJ��
=0.5mol����Һ̬˫��ˮ��Ӧ����Һ̬ˮʱ�ų�������0.5mol��817.75kJ/mol=408.875kJ��
��Ϊ:408.875KJ��
�۴˷�Ӧ���ڻ���ƽ������ͷŴ����ȺͿ��ٲ������������⣬����һ���ܴ���ŵ��Dz���Ϊ������ˮ���ǿ����ɷֲ�����ɻ�����Ⱦ��
��Ϊ:���ﲻ����ɻ�����Ⱦ��

 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д� ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д� ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д�