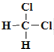��Ŀ����
����Ŀ��ʵ������ȼ�շ��ⶨij������(CxHyOzNp)�ķ�����ɣ�ȡw g�ð�������ڴ����г��ȼ�գ�����CO2��H2O��N2��������ͼ��ʾװ�ý���ʵ��(����̨�����С��ƾ��Ƶ�δ����)����ش��й����⣺
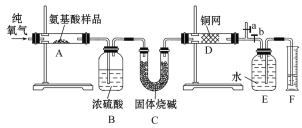
(1)ʵ����ֹˮ��a�ǹرյģ�b�ǿ����ġ���ʵ�鿪ʼʱ������Ҫ��a���رռ�b��ͨһ��ʱ��Ĵ�������������Ŀ����_____��
(2)����װ������Ҫ���ȵ���____(��װ�ô���)������ʱӦ�ȵ�ȼ___���ľƾ��ơ�
(3)װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ______��
(4)ʵ���в��N2�����ΪVmL(�ѻ���ɱ�״��)��Ϊȷ���˰�����ķ���ʽ������Ҫ�õ���������____(����ĸ)��
A.���ɶ�����̼��������� B.����ˮ������
C.ͨ����������� D.�ð��������Է�������
(5)�����װ��B��C������˳���ΪC��B����ʵ���Ŀ���ܷ�ﵽ?��������______��
���𰸡���װ���еĿ����ž� A��D D CxHyOzNp+(x+![]() -
-![]() )O2
)O2![]() xCO2+
xCO2+![]() H2O+
H2O+![]() N2 ABD ���ܣ���Ϊ�ռͬʱ����CO2��H2O�������壬ʹʵ����ȱ�ٱ�Ҫ�����ݣ���ȷ���ð�����ķ������
N2 ABD ���ܣ���Ϊ�ռͬʱ����CO2��H2O�������壬ʹʵ����ȱ�ٱ�Ҫ�����ݣ���ȷ���ð�����ķ������
��������
���������֪����ȼ�շ��ⶨij�ְ�����(CxHyOzNp)�ķ�����ɣ���˫��ˮ�����������ô�������CxHyOzNp�����ɶ�����̼��ˮ�͵�����������ͭ��δ��ȫ������̼Ԫ�������ɶ�����̼����Ũ��������ˮ���ü�ʯ�����ն�����̼����ͭ������δ��Ӧ������������ˮ��������õ����������Ϊ��ȷ��ø��ɷֵ�������ʵ�鿪ʼ����������װ���еĿ����ž���ͬʱӦ��������ˮ�������ն�����̼���ٳ�����������������������Ԫ���غ�ɼ����CxHyOzNp����ɣ��Դ˽����⡣
(1)�����еĵ�����������ţ�ʵ�鿪ʼʱ������Ҫ��a���رռ�b��ͨһ��ʱ��Ĵ��������Խ�װ���е�N2�ž�����������ʵ����ɸ��ţ�֮������ر�ֹˮ��a����b��
(2)������������ķ�Ӧ���Լ�ͭ���������ķ�Ӧ����Ҫ���ȣ������Ҫ���ȵ�װ����AD������ʱӦ�ȵ�ȼD���ľƾ��ƣ�����δ��Ӧ����������֤�����ռ�������ΪN2��
(3)��װ��A�а�����������ڼ���ʱ����������Ӧ����ѧ����ʽ�ǣ�CxHyOzNp+(x+![]() -
-![]() )O2
)O2![]() xCO2+
xCO2+![]() H2O+
H2O+![]() N2��
N2��
(4)��������ķ�����֪��Ϊ��ȷ���˰�����ķ���ʽ������ȷ����N2������⣬����õ��������Ħ�����������ɶ�����̼���������������ˮ���������ʺ���ѡ����ABD��
(5)�����װ���е�B��C����˳���ΪC��B����Ϊ��ʯ�ҽ�ͬʱ����ˮ������CO2���ò������߸��Ե�������������CԪ����HԪ�صĺ�����ʹʵ����ȱ�ٱ�Ҫ�����ݣ���ȷ���ð�����ķ�����ɡ�

����Ŀ���±��г�������������Ԫ�������ڱ��е�λ�á�
�� | ��A | 0 | ||||||
1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
2 | �� | �� | �� | �� | ||||
3 | �� | �� | �� | �� | ||||
�밴Ҫ��ش��������⣺
��1������������Ԫ���зǽ�������ǿ����________����Ԫ�ط��ţ���
��2��Ԫ������ԭ�ӽṹʾ��ͼ��_________������������������Ԫ����ɵĻ���������ʽ��_________��
��3��Ԫ������������̬�⻯����ȶ��Խ�ǿ����________________���ѧʽ����Ԫ��������������������Ӧ��ˮ��������Խ�ǿ����________________���ѧʽ����
��4��������span>��������Ԫ�ص�ԭ�Ӱ뾶�ɴ�С��˳����________________����Ԫ�ط��ţ���
��5��Ԫ����������ɵĻ�����Ļ�ѧ��������________________________��
��6��Ԫ����������������Ӧ��ˮ������Ԫ����������������Ӧ��ˮ���ﷴӦ�����ӷ���ʽ��________________
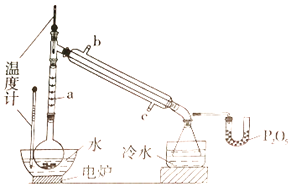
����Ŀ����������������������ˮ����Ѭ������ʵ���Һϳ�������������ԭ����װ�����£�
���������� | �״� | |
�ܽ��� | �����ѡ��״����ܣ���ˮ�� | ��ˮ���� |
�е�/�� | 68 | 64 |
������������״������Ĺ��е�Ϊ54�� | ||
Na2B4O7��10H2O+2H2SO4+16CH3OH![]() 2NaHSO4+4[(CH3O)3B+CH3OH]+17H2O
2NaHSO4+4[(CH3O)3B+CH3OH]+17H2O
ʵ�鲽�����£�
����Բ����ƿ�м���44.8g�״���19.1gNa2B4O7��10H2O (��ɰ��ʽ��Ϊ382)��Ȼ��������ŨH2SO4����;������ƿ�е�Һ��;ͨ������������һ��ʱ�䡣
���Ƚ���51~55�������,�ٽ���55~60������֡�
����������ֺϲ��������Ȼ��ƽ��������ֲ㣬�ϲ�Ϊ���������������롣
������øߴ�����������19.2g��
�ش��������⣺
��1��ͼ������a������Ϊ____________;ֱ����������ȴˮӦ��____________(����b������c��)�ӿڽ��롣
��2����ʵ����ȷ�ʽΪ____________���ŵ���____________��
��3�������Ȼ��������ֲ����ҪĿ����____________��
��4��U����P2O5��������_____________________________________��
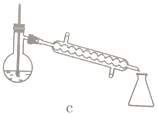
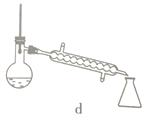
��5��������������ѡ��װ����ȷ����____________ (����)��Ӧ�ռ�____________������֡�


��6������ʵ��IJ�����____________��