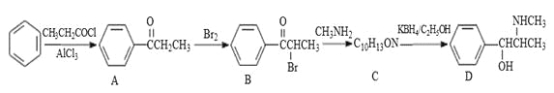
��Ŀ����
����Ŀ����ͭ������Ҫ����ͭԭ�ϣ� CuFeS2������ͭ����Ҫ������ʽ���ش��������⣺
(1)CuFeS2�д��ڵĻ�ѧ��������_________�����л�̬ԭ�ӻ����ӵļ۲�����ʾʽ��ȷ����_______�����ţ���
a.Fe2+��![]() b.Cu��
b.Cu��![]()
c.Fe3+��![]() d.Cu+��
d.Cu+��![]()
(2)�ڽϵ��¶��� CuFeS2��Ũ��������ʱ����������������ζ������X������
��X���ӵ����幹����________������ԭ���ӻ�����Ϊ______������________����Ǽ��ԡ����ԡ������ӡ�
��X�ķе��ˮ�͵���Ҫԭ����________��
(3)CuFeS2��������Ӧ����SO2��SO2����ԭ�ӵļ۲���Ӷ���Ϊ_____�����ۼ���������________��
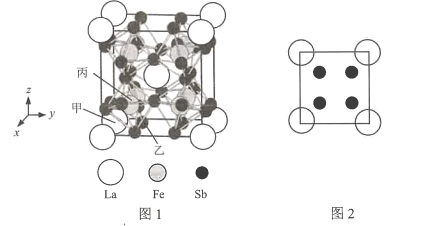
(4)�ķ���ϵCuFeS2�ľ����ṹ��ͼ��ʾ��
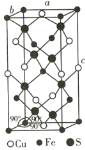
��Cu+����λ��Ϊ________��S2-����λ��Ϊ________��
����֪��a=b=0.524 nm��c=1.032 nm��NAΪ�����ӵ�������ֵ��CuFeS2������ܶ���________g��cm-3���г�����ʽ����
���𰸡����Ӽ� cd V�� sp3 ���� ˮ���Ӽ������� 3 ���������� 4 4 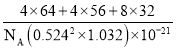
��������
(1)CuFeS2����Ϊ�ǽ���Ԫ�أ�����ͭΪ����Ԫ�أ�����֮���γɵĻ�ѧ��Ϊ���Ӽ���
A.Fe2+�ĵ����Ų�ʽΪ[Ar]3d6��A����
B.Cu�ĵ����Ų�ʽΪ[Ar]3d104s1��B����
C.Fe3+�ĵ����Ų�ʽΪ[Ar]3d5��C��ȷ��
D.Cu+�ĵ����Ų�ʽΪ[Ar]3d10��D��ȷ��
�ʺ���ѡ����CD��
(2)��������ζ������XΪH2S��
��H2S������S�����Թ¶Ե��ӣ����幹��ΪV�Σ�Ϊ���Է��ӡ�S���ӻ������Ϊ4���ӻ�����Ϊsp3�ӻ���
��H2O���Ӽ��������������˷���֮����������ã���H2S����֮��ֻ���ڷ��Ӽ������������H2S�ķе��ˮ�ͣ�
(3)SO2Ϊ���ۻ����Sԭ�Ӻ�����Oԭ�Ӽ�ֱ��γ�һ��������ͬʱ��ԭ�Ӻ���ԭ�Ӽ��γ�һ��4����������������SO2����Sԭ�ӵļ۲���Ӷ���Ϊ3�ԣ����ۼ��������У�������������
(4)�ٸ����������ͼ��֪��������Fe2+��ĿΪ��8��![]() +4��
+4��![]() +1=4��Cu+����ĿΪ��6��
+1=4��Cu+����ĿΪ��6��![]() +4��
+4��![]() =4��S2-��ĿΪ8�������ڹ���4��CuFeS2��a=b=0.524 nm��c=1.032 nm��������ܶ���=
=4��S2-��ĿΪ8�������ڹ���4��CuFeS2��a=b=0.524 nm��c=1.032 nm��������ܶ���=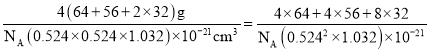 g/cm3��
g/cm3��

����Ŀ����(Ge)�ǵ��͵İ뵼��Ԫ�أ��ڵ��ӡ����ϵ�����Ӧ�ù㷺���ش��������⣺
(1)Ge��C��ͬ��Ԫ�أ�Cԭ��֮������γ�˫������������Geԭ��֮�������γ�˫������������ԭ�ӽṹ���۵�/��Ƕȷ�����ԭ����______________��
(2)�Ƚ�������±������۵�ͷе㣬������仯���ɼ�ԭ��____________��
GeCl4 | GeBr4 | Gel4 | |
�۵�/�� | -49.5 | 26 | 146 |
�е�/�� | 83.1 | 186 | Լ400 |
(3)�����ԭCO2�Ʊ�CH4��Ӧ�У���״����Zn2GeO4�Ǹ÷�Ӧ�����ô�����Zn��Ge��O�縺���ɴ���С��˳����______________��
(4)Ge�������н��ʯ�ͽṹ������Geԭ�ӵ��ӻ���ʽΪ__________����֮����ڵ���������________��
(5)��������������Ҫ�أ�
��ԭ�������������ʾ�����ڲ���ԭ�ӵ����λ�á���ͼΪGe�����ľ���������ԭ���������AΪ��0��0��0����BΪ(![]() ��0��
��0��![]() )��C(
)��C(![]() ��0��
��0��![]() )����Dԭ�ӵ��������Ϊ_________��
)����Dԭ�ӵ��������Ϊ_________��
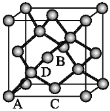
�ھ������������������Ĵ�С����״����֪Ge�����ľ�������a=565.76 pm�����ܶ�Ϊ_______ g��cm3���г�����ʽ���ɣ���
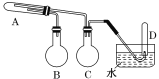
����Ŀ���������Ϊ5mL�ļס��ҡ�������Һ���������Թܱڻ��������Թ��У���������������ͼ��ʾ��ʵ��������ס��ҡ�������Ͽ����ǣ� ��

ѡ�� | �� | �� | �� |
A | 1�� | ˮ | �� |
B | �屽 | Һ�� | �Ҵ� |
C | ˮ | ����ϩ | ��ˮ |
D | �Ҵ� | ���� | �������� |
A.AB.BC.CD.D