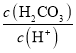��Ŀ����
����Ŀ�����Ļ�������ҽҩ��������������Ҫ���ã���ʶ�����仯��������Ҫ���塣
��1����̬��ԭ���У�����ռ�ݵ�����ܼ�����Ϊ_____________��
��2���о������������������������δ�ɶԵ���ʱ���û�������д��ԡ��������ʿ�����¼�����ŷ۵���__________��
A��V2O5 B��Fe2O3 C��ZnO D��CrO2
��3����֪Cr3+��ˮ��Һ�еĴ�����ʽΪ[Cr(H2O)6��3+���ڲ�ͬ�����£��ɴ�CrCl3ˮ��Һ�л����ɫ������ɫ����ɫ�Ȳ�ͬ��ɫ��������ʵ��ʽ��ΪCrCl36H2O����ȡ����ɫ�����0.1mol����������AgNO3��Һ�������ˡ�ϴ�ӡ������28.7g��������û������еĻ�ѧ��������___________��д��������ɫ�����ĵ��뷽��ʽ��________��
��4�������£��Ȼ�����(CrO2Cl2)�ǰ���ɫҺ�壬����CCl4��CS2���л��ܼ����ܡ�
�ٹ����Ȼ���������______�����������������Ǽ����������ӣ��ж�������______��
�ڵȵ������Ǿ�����ͬ�ļ۵�������ԭ�����ķ��ӻ����ӡ�д��һ����CCl4���ӻ�Ϊ�ȵ�����������ӣ�_______���ѧʽ����д��CS2���ӵĵ���ʽ��____________��
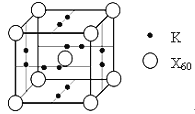
��5��������(CrN)�ڳ�������������������Ӧ��ǰ�����侧��ṹ�������Ȼ�����ͬ��
�ٵ��������۵���Ȼ��Ƹߵ���Ҫԭ����______��
����Cr3+���ڽ���N3�C��________����
����֪Cr3+��N3�C�뾶�ֱ�Ϊapm��bpm����Cr3+��N3�C���ǽ��ܽӴ��ĸ���С��NA���������ӵ�������ֵ��������ܶ�Ϊ��gcm�C3����CrN��Ħ������Ϊ______�����ú�a��b��NA��������ʽ��ʾ��
���𰸡�3d BD ���Ӽ��������ԣ����ۼ�����λ�� [Cr(H2O)5C1]C12=[Cr(H2O)5C1]2++2Cl- �Ǽ��� ������������ԭ����CC14��CS2Ϊ�Ǽ��Է��ӣ�����CrO2C12Ϊ�Ǽ��Է��� PO43�C��SO42�C��C1O4�C ![]() ����������������������϶࣬�����ܽϴ� 8
����������������������϶࣬�����ܽϴ� 8 ![]()
��������
��5���۸��������Ϣ����Ͼ�����������ʽ����m=��V����![]() ���ɵã�
���ɵã�![]() ���ɴ˿�֪��Ҫ����CrN��Ħ������������㾧������������������������������ݴ˽��з�����⡣
���ɴ˿�֪��Ҫ����CrN��Ħ������������㾧������������������������������ݴ˽��з�����⡣
��1��CrԪ�صĻ�̬ԭ�ӵĵ����Ų�ʽΪ��Ar��3d54s1��3d�ܼ���4s�ܼ������ܼ�������3d��������4s�ģ����Ի�̬��ԭ���е���ռ�ݵ�����ܼ�����Ϊ3d����Ϊ��3d��
��2������Ϣ֪����������д�����Ҫ������������δ�ɶԵ��ӣ��ݴ�
A��V2O5�з�Ϊ+5�ۣ���Ӧ�����ӵĵ����Ų�ʽΪ1s22s22p63s23p6����������û��δ�ɶԵ��ӣ�����V2O5û�д��ԣ�A�����
B��Fe2O3����Ϊ+3�ۣ���Ӧ�����ӵĵ����Ų�ʽΪ1s22s22p63s23p63d5��3d5��5��δ�ɶԵ��ӣ�����Fe2O3�д��ԣ�B����ȷ��
C��ZnO��пΪ+2�ۣ���Ӧ�����ӵĵ����Ų�ʽΪ1s22s22p63s23p63d10����������û��δ�ɶԵ��ӣ�����ZnOû�д��ԣ�C�����
D��CrO2�и�Ϊ+4�ۣ���Ӧ�����ӵĵ����Ų�ʽΪ1s22s22p63s23p63d2��3d2��2��δ�ɶԵ��ӣ�����CrO2�д��ԣ�D����ȷ��
����BD��
��3����0.1mol����ɫ������м���������AgNO3��Һ�������ˡ�ϴ�ӡ������28.7g��������n(AgCl)=![]() ����1mol����ɫ������м���������AgNO3�ܲ���2molAgCl��������Ϊ������ڽ粻��������ӣ���1mol����ɫ���������3molCl-����������ɫ���������Cl-���ڽ��2���������2��Cl-���ڽ�1��Cl-���ٸ�������֪Cr3+��ˮ��Һ�еĴ�����ʽΪ[Cr(H2O)6��3+����֪Cr����λ��Ϊ6�������ڽ绹��5��H2O����绹��1��H2O���������ɫ�����ĽṹʽΪ[Cr(H2O)5C1]C12��H2O��������������ڽ����磬�ܵ�������ӣ����Ժ������Ӽ���H2O�д��ڣ����ԣ����ۼ����ڽ��H2O��Cl-��Cr3+֮�������λ��������뷽��ʽΪ��[Cr(H2O)5C1]C12=[Cr(H2O)5C1]2++2Cl-����Ϊ�����Ӽ��������ԣ����ۼ�����λ����[Cr(H2O)5C1]C12=[Cr(H2O)5C1]2++2Cl-��
����1mol����ɫ������м���������AgNO3�ܲ���2molAgCl��������Ϊ������ڽ粻��������ӣ���1mol����ɫ���������3molCl-����������ɫ���������Cl-���ڽ��2���������2��Cl-���ڽ�1��Cl-���ٸ�������֪Cr3+��ˮ��Һ�еĴ�����ʽΪ[Cr(H2O)6��3+����֪Cr����λ��Ϊ6�������ڽ绹��5��H2O����绹��1��H2O���������ɫ�����ĽṹʽΪ[Cr(H2O)5C1]C12��H2O��������������ڽ����磬�ܵ�������ӣ����Ժ������Ӽ���H2O�д��ڣ����ԣ����ۼ����ڽ��H2O��Cl-��Cr3+֮�������λ��������뷽��ʽΪ��[Cr(H2O)5C1]C12=[Cr(H2O)5C1]2++2Cl-����Ϊ�����Ӽ��������ԣ����ۼ�����λ����[Cr(H2O)5C1]C12=[Cr(H2O)5C1]2++2Cl-��
��4���ٸ�����������ԭ����CCl4��CS2Ϊ�Ǽ��Է��ӣ�����CrO2Cl2Ϊ�Ǽ��Է��ӣ���Ϊ���Ǽ��ԣ�������������ԭ����CCl4��CS2Ϊ�Ǽ��Է��ӣ�����CrO2Cl2Ϊ�Ǽ��Է��ӣ�
�ڵȵ������Ǿ�����ͬ�ļ۵�������ԭ�����ķ��ӻ����ӣ�CCl4����AX4ͨʽ��CCl4����5��ԭ�ӡ�32���۵��ӣ���PO43-��SO42-��ClO4-��Щ���ӻ�Ϊ�ȵ����壻CS2�ĵ���ʽΪ��![]() span>����Ϊ��PO43�C��SO42�C��C1O4�C��
span>������PO43�C��SO42�C��C1O4�C��![]() ��
��
��5����ͨ���������������Խ�࣬�����ܾ�Խ���۷е��Խ�ߡ���Ϊ������������������������϶࣬�����ܽϴ�
���������Ϣ������������ṹ�������Ȼ�����ͬ���ݴ˷����������Cr3+������N3-�ľ���Ϊ���ڽ����룬�ö����Cr3+Ϊ8�����������У�������ö����Cr3+���ڽ���N3-��8������Ϊ��8��
����֪Cr3+��N3-�뾶�ֱ�Ϊapm��bpm��Cr3+��N3-���ǽ��ܽӴ��ĸ���С�����Ծ����ı߳�Ϊ(2a+2b)pm=![]() ������Cr3+λ��8�������6����������ϣ�����һ����������4��Cr3+��N3-λ��12�����Ϻ�1�����ģ�����һ����������4��N3-����һ����������4��CrN��һ��������������4��CrN����������CrN��Ħ������ΪM����һ������������m=
������Cr3+λ��8�������6����������ϣ�����һ����������4��Cr3+��N3-λ��12�����Ϻ�1�����ģ�����һ����������4��N3-����һ����������4��CrN��һ��������������4��CrN����������CrN��Ħ������ΪM����һ������������m=![]() �����������������ھ����ܶ��뾧������ij˻�����
�����������������ھ����ܶ��뾧������ij˻�����![]() �����������V=
�����������V=![]() �����Ծ��������Ĺ�ϵʽ���£�
�����Ծ��������Ĺ�ϵʽ���£�![]() ��������M=
��������M=![]() ������
������ ![]() ��
��

����Ŀ�������½�������ʵ�飬����ʵ��������������õ��Ľ�����ȷ����
ѡ�� | ʵ����������� | ���� |
A | ��X��Һ�еμ� | X��Һ��һ������ |
B | ��Ũ�Ⱦ�Ϊ0.05mol��L-1�� |
|
C | ��2mLŨ�Ⱦ�Ϊ0.05mol��L-1�� |
|
D | �� | ��� |
A.AB.BC.CD.D
����Ŀ��![]() ��Ӧ���̵������仯��ͼ��ʾ����ش��������⣺
��Ӧ���̵������仯��ͼ��ʾ����ش��������⣺
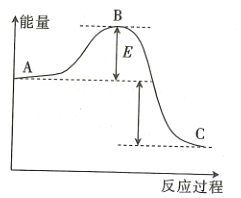
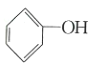
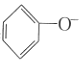
(1)�÷�Ӧ��______________��������������������������Ӧ��
(2)��![]() ���÷�Ӧ�Ĵ�����A��C������������Ƿ�仯?_____________�������仯����������������������____________________��
���÷�Ӧ�Ĵ�����A��C������������Ƿ�仯?_____________�������仯����������������������____________________��
(3)�����Ϊ2L�ĺ����ܱ�������ͨ��2mol![]() ��3mol
��3mol![]() ����������Ӧ��10min��
����������Ӧ��10min��![]() �����ʵ����仯���±���
�����ʵ����仯���±���
��Ӧʱ�䣨min�� | 0 | 3 | 5 | 7 | 10 |
| 0 | 0.2 | 0.3 | 0.38 | 0.38 |
�������¶ȣ���Ӧ����______________������������������С������
��������Ӧ��7minʱ_______________�������ﵽ������δ�ﵽ����ƽ��״̬��
��![]() min�ڣ���
min�ڣ���![]() ��ʾ�÷�Ӧ�ķ�Ӧ����Ϊ_______________mol��L-1��min-1��
��ʾ�÷�Ӧ�ķ�Ӧ����Ϊ_______________mol��L-1��min-1��