��Ŀ����
20��ij��ѧ��ȤС��ⶨijFe2��SO4��3��Ʒ��ֻ������FeCl2���ʣ�����Ԫ�ص�����������������ʵ�鲽����в������ٳ�ȡa g��Ʒ�������ձ��У�
�ڼ���50mL 1.0mol/Lϡ�����һ����������ˮ��ʹ��Ʒ�ܽ⣬Ȼ��ȷ���Ƴ�250.00mL��Һ��
����ȡ25.00mL���������õ���Һ�������ձ��У�������������ˮ��ʹ��Ӧ��ȫ��
�ܼ��������ˮ����ֽ��裬ʹ������ȫ��
�ݹ��ˣ�ϴ�ӳ�����
������ת�Ƶ�ij�����ڣ����ȡ����裬ֱ�������ɺ��ɫȫ����Ϊ����ɫ���ڸ���������ȴ�����º�����
�ߡ�
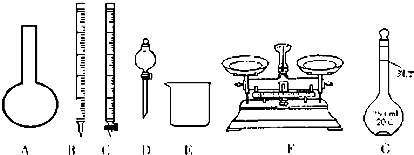
����������������ش�
��1����ͼ��ʾ�����У���ʵ�鲽��٢ڢ��б����õ���������E��CFG������ĸ����
��2��������У�����50mL��1.0mol/LϡH2SO4������Ͳ��ȡ98%���ܶ�1.84g/cm3����ŨH2SO4���Ϊ2.7 mL�������ƹ����У�ijѧ���۲춨��ʱ���ӣ�������Һ��Ũ�Ȼ�ƫ�ߣ��ƫ�ߡ�����ƫ�͡�����Ӱ�족����
��3����Ʒ�е�����Fe2+�н�ǿ�Ļ�ԭ�ԣ���ɲ���ƽ���з�Ӧ�����ӷ���ʽ��Fe2++��ClO2+����=��Fe3++��Cl-+��H2O
�������Ϸ���������ʵ����������ˮ��ΪClO2ʱ����ʵ������ɲ�Ӱ�죨�ƫ��ƫС����Ӱ�족���������ʵ�����ClO2��Cl2�ĵ���ת����֮��Ϊ5��2��
��4����������ڸ���������ȴ����ⶨ����Ԫ�ص�����������ƫ���ƫ��ƫС����Ӱ�족����������������W1g�����������������������W2 g������Ʒ����Ԫ�ص���������Ϊ$\frac{0.7��{W}_{2}-{W}_{1}��}{a}$��100%��
���� ��1�����ݸ�����������ѡȡ������
��2������������Һϡ���������ʵ����������������Һ�����
��3������������ԭ��Ӧ�����غ㡢ԭ���غ���ƽ���ӷ���ʽ����ԭ��һ������Ҫ�������������������������ƣ���ԭ��ʧ������һ�����������ı䲻Ӱ������������������������ǵõ�������֮�ȣ�
��4����������ڸ���������ȴ�������տ����е�ˮ����������������������ⶨ����Ԫ�ص���������ƫ�ߣ���������ֱ�������ɺ��ɫȫ����Ϊ����ɫ��������������صı������γ�������������0.1g��
������Ԫ�������غ㣬������ɫ���壨 Fe2O3���е���������Ʒ�������������������Ĺ�ʽ�����Ԫ�ص�����������
��� �⣺��1������ҩƷ����ƽ���Ȼ������Ȼ�������ˮ��Һ�������ԣ�����ȷ��ȡ25.00mL���������õ���Һ����ʽ�ζ��ܣ�����һ�����ʵ���Ũ�ȵ���Һ������ƿ���ʴ�Ϊ��CFG��
��2������50mL��1.0mol/LϡH2SO4��Ҫ98%���ܶ�1.84g/cm3����ŨH2SO4���Ϊ��ΪVml������ϡ��ǰ����Һ�������ʵ������䣬$\frac{Vml��1.84g/ml��98%}{98g/mol}$=0.05L��1.0mol/L��V=2.7ml�������ƹ����У�ijѧ���۲춨��ʱ���ӣ�ˮδ���뵽�̶��ߣ���ҺŨ������
�ʴ�Ϊ��2.7��ƫ�ߣ�
��3����Ӧ�У��������ӱ仯Ϊ�����ӣ������������Ԫ�ػ��ϼ۴�+4�۱仯Ϊ-1�ۣ��仯5�ۣ�����ת����С������Ϊ5�����ݵ����غ��ԭ���غ���ƽ�õ����ӷ���ʽΪ��5Fe2++ClO2+4 H+�T5Fe3++Cl-+2H2O���������Ϸ���������ʵ����������ˮ��ΪClO2ʱ��ʵ����Ӱ�죬��ͬ������������ͬ��ͬ����ԭ�����ʱ�������Ҫʧȥ������ͬ�������ʵ�����ClO2��Cl2������Ч��֮��ΪΪת�Ƶ�����֮�ȣ�ClO2��Cl-��5e-��Cl2��2Cl-��2e-������1molClO2��Cl2�ĵ���ת��֮��Ϊ5��2��
�ʴ�Ϊ��5��1��4H+��5��1��2����Ӱ�죻5��2��
��4����������ڸ���������ȴ�������տ����е�ˮ����������������������ⶨ����Ԫ�ص���������ƫ��
����Ԫ�������غ㣬������ɫ�����е���������Ʒ������Fe2O3����Ԫ�ص�����Ϊ��W2-W1��g��$\frac{112}{160}$����Ʒ����Ԫ�ص�����������$\frac{0.7��{W}_{2}-{W}_{1}��}{a}$��100%��
�ʴ�Ϊ��ƫ��$\frac{0.7��{W}_{2}-{W}_{1}��}{a}$��100%��
���� ���⿼����ʵ��̽��������ʵ��ⶨ����Һ���ƹ��̣���������������������ԭ��Ӧ��ƽ�ǽ���ؼ���ע��ʵ��ⶨ��Һ����ı仯����Ŀ�Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д�| A�� | ��ϡ���ᴦ����Ƭ | B�� | ��ϡ���ᴦ����Ƭ | ||
| C�� | ��Ũ���ᴦ����Ƭ | D�� | ��Ũ���ᴦ����Ƭ |
| A�� | HOCH2CH2OH | B�� | HOCH2CH��OH��CH3 | C�� | CH3CH2CH2OH | D�� | CH3CH��OH��CH��OH��CH3 |
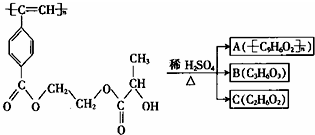
| A�� | M��A����ʹ����KMnO4��Һ����ˮ��ɫ | |
| B�� | B��C3H6O3���ܷ�����ȥ��Ӧ��������Ӧ | |
| C�� | 1mol M�������ȵ��ռ���Һ��Ӧ����������2n mol NaOH | |
| D�� | A��B��C��1mol�ֱ������������Ʒ�Ӧ���ų���������ʵ���֮��Ϊ1��2��2 |
| A�� | ��ˮ������ˮ���Թ�����NO2���ɿ���Һ��������������һ������O2��ʹҺ����������Թ� | |
| B�� | ��ˮ������NaOH��Һ���Թ�����Cl2���ɿ���Һ���������Թ��л���ɫ��ȥ | |
| C�� | ��ˮ������ˮ�����м�����ɫʯ��Թ�����SO2���ɿ���Һ����������Ϊ��ɫ | |
| D�� | ��ˮ������ˮ�����м��η�̪���Թ�����NH3���ɿ���Һ���������ʺ�ɫ |
| A�� | ԭ�Ӱ뾶�ɴ�С��˳���ǣ�C��B��A | |
| B�� | AxBy��A�Ļ��ϼ۲�����Ϊ+1�� | |
| C�� | C��B�γɵĻ���������ˮ���ܵõ�����B | |
| D�� | A��B��Ԫ�ص���̬�⻯��������Ӧ |
| A�� | Ԫ��Y��Z��W���Ӿ�����ͬ�ĵ��Ӳ�ṹ����뾶�������� | |
| B�� | Ԫ��X��Ԫ��Y�����γ����ֹ��ۻ�����������ֻ�����ֻ��һ�����͵Ĺ��ۼ� | |
| C�� | Ԫ��Y��R�ֱ���Ԫ��X�γɵĻ���������ȶ��ԣ�XmY��XnR | |
| D�� | Ԫ��W��R������������Ӧˮ���ﶼ��ǿ�� |
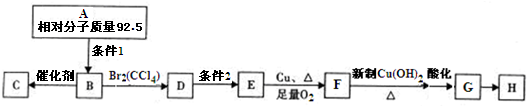
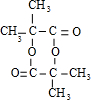 ��
�� ��
��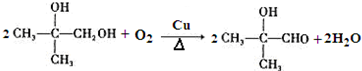 ��
�� ���ĺϳ�·��Ϊ
���ĺϳ�·��Ϊ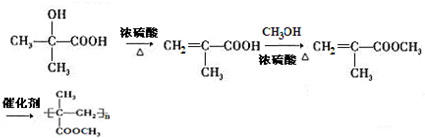 ��
��