��Ŀ����
�����ʽṹ�����ʡ�
X��Y��Z��M��N��QΪԪ�����ڱ�ǰ�����ڵ�����Ԫ�ء�����Xԭ�Ӻ����M����ֻ�����ԳɶԵ��ӣ�Yԭ�Ӻ����L���������K���������Z�ǵؿ��ں�����������������ߵ�Ԫ�أ�M���ڲ��������������������9����N��ԭ��������MС1, Q��Ԫ�����ڱ��ĸ�Ԫ���е縺�������ش��������⣺
��1��XԪ�������ڱ��е�λ���� ���� ��Ԫ�أ������������ӵĵ����Ų�ͼΪ ��
��2��XZ2���ӵ�����ṹ�� ��YZ2������Y���ӻ��������Ϊ ����ͬ������������ˮ�е��ܽ�Ƚϴ���� ��д����ʽ���������� ��
��3������Ԫ��N���ε���ɫ��ӦΪ ɫ����������ζ����Է�����ɫ��Ӧ����ԭ���� ��
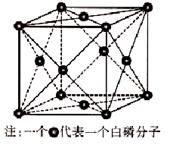
��4��Ԫ��M��Ԫ��Q�γɾ���ṹ��ͼ��ʾ�����侧���߳�Ϊa pm����aλ����bλ��֮��ľ���Ϊ_______pm��ֻҪ������ʽ����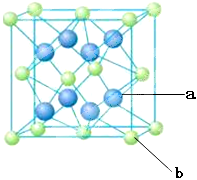
��1���������ڢ�A�� P 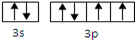
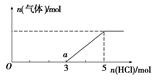
��2��V sp SO2 SO2Ϊ���Է��ӣ�CO2Ϊ�Ǽ��Է��ӣ�H2OΪ�����ܼ������Է��������ڼ����ܼ�����SO2���ܽ�Ƚϴ�
��3���� �����ɽϸ��ܼ�ԾǨ���ϵ��ܼ�ʱ���Թ����ʽ�ͷ�������
��4��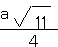
����

��14�֣�Ԫ�����ڱ���ѧϰ��ѧ����Ҫ���ߣ���������������Ϣ���ɣ������ǰ��ֶ�����Ԫ�ص������Ϣ����֪���ԭ�Ӱ뾶Ϊ0.089nm��
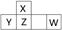
| Ԫ�ش��� | A | B | C | D | E |
| ԭ�Ӱ뾶/nm | 0.16 | 0.143 | 0.102 | 0.099 | 0.074 |
| ��Ҫ���ϼ� | +2 | +3 | +6��-2 | -1 | -2 |
��1��BԪ����Ԫ�����ڱ��е�λ��___________________����������Ԫ�ص�����������Ӧ��ˮ������������ǿ����__________��A���ӵĽṹʾ��ͼ_______________��
��2���õ���ʽ��ʾA��D�γɻ�����Ĺ��̣�____________________________________��H��E�γ�ԭ�Ӹ�����Ϊ1��1�Ļ�����ĵ���ʽΪ___________�����еĻ�ѧ������Ϊ____________��C2D2�ĵ���ʽΪ______________________��
��3������˵����˵��D�ķǽ����Ա�Cǿ��ѡ��____________
��H2CO4��HDO�ȶ���HDO4��H2CO4����ǿ��C2-��D-�ױ�������HD��H2C�ȶ���ͭ��HD����Ӧ��������ŨH2CO4��Ӧ������D2��������FeD3������C��������FeC��Cԭ����Dԭ�ӵ��Ӳ�����ͬ��Dԭ�Ӱ뾶С��Cԭ�ӡ�
A��ȫ�� B���ڢۢܢޢ� C���٢ڢܢݢ� D����������
��4��A��B��C��D��E�γɵļ����Ӱ뾶�ɴ�С��˳��Ϊ_______________________�����þ������ӷ��ű�ʾ��
��5��C������H������������Ӧˮ�����ڼ����������ܷ�����Ӧ������3mol��C���뷴Ӧ��ת��4NA�ĵ��ӣ���д�����ӷ�Ӧ����_______________________________________���������뻹ԭ��������֮��_____________________��
���и�������У�����ԭ���ӻ���������Ͳ���ͬ����
| A��CO2��SO2 | B��CH4��NH3 | C��SO3��BF3 | D��H2S��CCl4 |
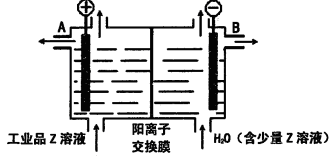
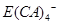 ����ʽ���ڣ�д��E����Z��Һ�����ӷ���ʽ��_________________________________________.
����ʽ���ڣ�д��E����Z��Һ�����ӷ���ʽ��_________________________________________.