��Ŀ����
A��B��C��D��E��F��Ϊ������Ԫ�أ�ԭ��������������AԪ��ԭ�Ӻ��������ӣ�BԪ��ԭ�Ӻ��������������Ǵ�����������2����CԪ���ǵؿ��ﺬ������Ԫ�أ�D�Ƕ�����Ԫ���н�������ǿ��Ԫ�أ�E��DԪ�ص�������֮��Ϊ27������������֮��Ϊ5��FԪ�ص�����������ˮ����Ϊ��ǿ�ᣮ
��1���ƶ�BԪ����Ԫ�����ڱ��е�λ�ã��� ���� �壮
��2������FԪ�ص�ԭ�ӽṹʾ��ͼ��
��3��A��EԪ���γɻ�����û��������18���ӣ��������ʽΪ��
��4��д��A��CԪ���γ�10���ӵ�һ�����Ļ�ѧʽ��
��5��C��DԪ�ؿ��γɵ���ɫ�ķ�ĩ��B��CԪ�ؿ��γ��ɼ��Լ����ɵķǼ��Է��ӣ�����������֮�䷢����Ӧ�Ļ�ѧ����ʽΪ ��
��1���ƶ�BԪ����Ԫ�����ڱ��е�λ�ã���
��2������FԪ�ص�ԭ�ӽṹʾ��ͼ��
��3��A��EԪ���γɻ�����û��������18���ӣ��������ʽΪ��
��4��д��A��CԪ���γ�10���ӵ�һ�����Ļ�ѧʽ��
��5��C��DԪ�ؿ��γɵ���ɫ�ķ�ĩ��B��CԪ�ؿ��γ��ɼ��Լ����ɵķǼ��Է��ӣ�����������֮�䷢����Ӧ�Ļ�ѧ����ʽΪ
������A��B��C��D��E��F��Ϊ������Ԫ�أ�ԭ��������������AԪ��ԭ�Ӻ��������ӣ���AΪHԪ�أ�BԪ��ԭ�Ӻ��������������Ǵ�����������2����ԭ��ֻ����2�����Ӳ㣬����������Ϊ4����BΪ̼Ԫ�أ�CԪ���ǵؿ��ﺬ������Ԫ�أ���CΪ��Ԫ�أ�D�Ƕ�����Ԫ���н�������ǿ��Ԫ�أ���DΪNa��E��DԪ�ص�������֮��Ϊ27����E��������=27-11=16����EΪ��Ԫ�أ�FԪ�ص�����������ˮ����Ϊ��ǿ�ᣬ��FΪCl���ݴ˽��
����⣺A��B��C��D��E��F��Ϊ������Ԫ�أ�ԭ��������������AԪ��ԭ�Ӻ��������ӣ���AΪHԪ�أ�BԪ��ԭ�Ӻ��������������Ǵ�����������2����ԭ��ֻ����2�����Ӳ㣬����������Ϊ4����BΪ̼Ԫ�أ�CԪ���ǵؿ��ﺬ������Ԫ�أ���CΪ��Ԫ�أ�D�Ƕ�����Ԫ���н�������ǿ��Ԫ�أ���DΪNa��E��DԪ�ص�������֮��Ϊ27����E��������=27-11=16����EΪ��Ԫ�أ�FԪ�ص�����������ˮ����Ϊ��ǿ�ᣬ��FΪCl��
��1��BΪ̼Ԫ�أ�ԭ�Ӻ�����2�����Ӳ㣬�������4�����ӣ�λ�����ڱ��ڶ����ڵڢ�A�壬�ʴ�Ϊ��������A��
��2��FΪ��Ԫ�أ�ԭ�Ӻ�����17�����ӣ���3�����Ӳ㣬����������Ϊ7����ԭ�ӽṹʾ��ͼΪ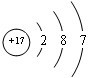 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��3��H��SԪ���γɾ���18���ӻ�����û�����ΪH2S��Sԭ����Hԭ��֮���γ�1�Թ��õ��Ӷԣ��������ʽΪ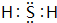 ���ʴ�Ϊ��
���ʴ�Ϊ��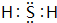 ��
��
��4��AΪ��Ԫ�ء�CΪ��Ԫ�أ���Ԫ������Ԫ���γ�10���ӵ�����H2O��H3+O��OH-���ʴ�Ϊ��H2O��H3+O��OH-��
��5��O��NaԪ�ؿ��γɵ���ɫ�ķ�ĩΪNa2O2��C��OԪ�ؿ��γ��ɼ��Լ����ɵķǼ��Է���ΪCO2������������֮�䷢����Ӧ�Ļ�ѧ����ʽΪ2Na2O2+2CO2=2Na2CO3+O2��
�ʴ�Ϊ��2Na2O2+2CO2=2Na2CO3+O2��
��1��BΪ̼Ԫ�أ�ԭ�Ӻ�����2�����Ӳ㣬�������4�����ӣ�λ�����ڱ��ڶ����ڵڢ�A�壬�ʴ�Ϊ��������A��
��2��FΪ��Ԫ�أ�ԭ�Ӻ�����17�����ӣ���3�����Ӳ㣬����������Ϊ7����ԭ�ӽṹʾ��ͼΪ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����3��H��SԪ���γɾ���18���ӻ�����û�����ΪH2S��Sԭ����Hԭ��֮���γ�1�Թ��õ��Ӷԣ��������ʽΪ
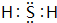 ���ʴ�Ϊ��
���ʴ�Ϊ��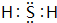 ��
����4��AΪ��Ԫ�ء�CΪ��Ԫ�أ���Ԫ������Ԫ���γ�10���ӵ�����H2O��H3+O��OH-���ʴ�Ϊ��H2O��H3+O��OH-��
��5��O��NaԪ�ؿ��γɵ���ɫ�ķ�ĩΪNa2O2��C��OԪ�ؿ��γ��ɼ��Լ����ɵķǼ��Է���ΪCO2������������֮�䷢����Ӧ�Ļ�ѧ����ʽΪ2Na2O2+2CO2=2Na2CO3+O2��
�ʴ�Ϊ��2Na2O2+2CO2=2Na2CO3+O2��
������������Ԫ���ƶ�Ϊ���壬�����������Ų�ʽ��ԭ�ӽṹʾ��ͼ������ʽ����Ԫ�ػ��������ʵȣ��Ƕ���ѧ֪ʶ���ۺ����ÿ��飬�ѶȲ���ע�����֪ʶ���������գ�

��ϰ��ϵ�д�
 �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�
�����Ŀ
 [��ѧ/ѡ��/���ʽṹ������]A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������Ԫ�ض�Ӧ�ĵ��ʾ�Ϊ���壮A��C��E��Ԫ�ص�ԭ�Ӻ����ֻ��2��δ�ɶԵ��ӣ�B��EԪ�ص�ԭ������֮�͵���C��DԪ�ص�ԭ������֮�ͣ�
[��ѧ/ѡ��/���ʽṹ������]A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������Ԫ�ض�Ӧ�ĵ��ʾ�Ϊ���壮A��C��E��Ԫ�ص�ԭ�Ӻ����ֻ��2��δ�ɶԵ��ӣ�B��EԪ�ص�ԭ������֮�͵���C��DԪ�ص�ԭ������֮�ͣ�