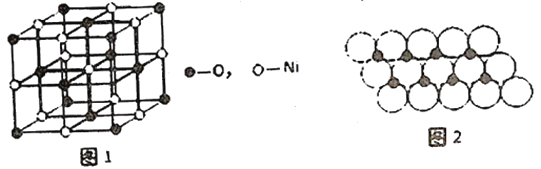��Ŀ����
����Ŀ��X��Y��Z��QΪ�����ڷǽ���Ԫ�أ�R�dz�����Ԫ�ء�Xԭ�ӵĵ���ռ��2�����Ӳ���ԭ���гɶԵ�������δ�ɶԵ�������2����Y�Ļ�̬ԭ����7�ֲ�ͬ�˶�״̬�ĵ��ӣ�ZԪ���ڵؿ��к�����ࣻQ�ǵ縺������Ԫ�أ�R��ֻ���������Ӳ�����ȫ�������ӡ�
��ش��������⣺(����ʱ��X��Y��Z��Q��R������Ӧ��Ԫ�ط��ű�ʾ)
��1��R�Ļ�̬ԭ�ӵĵ����Ų�ʽΪ________��
��2��X��Y��Z����Ԫ�ص�һ�����ܴӴ�С˳��Ϊ______________________��
��3����֪Y2Q2���Ӵ���������ʾ�����ֽṹ(���ģ�ͣ����߲�һ����������)��

�ٸ÷���������Yԭ��֮��ļ��������ȷ����________��
A����1������
B��1��������2������
C��1��������1������
D����2������
�ڸ÷�����Yԭ�ӵ��ӻ���ʽ��______________________��
���𰸡� 1s22s22p63s23p63d104s1(��[Ar]3d104s1) N>O>C C sp2
��������������Ҫ����ԭ�ӽṹ��Ԫ�����ʡ�
�������֪XΪ̼Ԫ�أ�YΪ��Ԫ�أ�ZΪ��Ԫ�أ�QΪ��Ԫ�أ�RΪͭԪ�ء�
(1)CuΪ29��Ԫ�أ������Ų�ʽΪ1s22s22p63s23p63d104s1��[Ar]3d104s1��
(2)C��N��O����Ԫ�صĵ�һ�������ɴ�С��˳��ΪN>O>C��
(3)��N2F2�����ֽṹ��֪����ṹʽΪ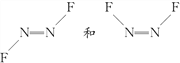 ��������ԭ����˫����ʽ�ɼ�������1��������1��������C����ȷ����ԭ����sp2�ӻ���
��������ԭ����˫����ʽ�ɼ�������1��������1��������C����ȷ����ԭ����sp2�ӻ���

 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�����Ŀ������Ǧ������Ǧ���ء���ά���ؼ���Ϳ�Ϸ����Լ�����ҵ��ͨ���÷�Ǧ��(��Ҫ�ɷ�ΪPbS)��������Ǧ�������������£�

��֪��
��25�棬Ksp(PbS)=1.0��10-28��Ksp(PbCl2)=1.6��10-5
��PbCl2(s)+2Cl-(aq)![]() PbCl42-(aq) ��H>0
PbCl42-(aq) ��H>0
��Fe3+������������ʽ��ʼ����ʱ��pHֵΪ1.9
(1)����Ksp(PbS)��Ksp(PbCl2)��PbS+2HCl![]() PbCl2+H2S�ķ�Ӧ�̶Ⱥ�С������FeCl3������Ӧ�̶ȣ�ԭ����____________________������ٷ�Ӧ�����пɹ۲쵽�е���ɫ�������ɣ��ܷ�Ӧ�����ӷ���ʽΪ_____________________���ò����������Һ��pH<1.9����ҪĿ����_______________________��
PbCl2+H2S�ķ�Ӧ�̶Ⱥ�С������FeCl3������Ӧ�̶ȣ�ԭ����____________________������ٷ�Ӧ�����пɹ۲쵽�е���ɫ�������ɣ��ܷ�Ӧ�����ӷ���ʽΪ_____________________���ò����������Һ��pH<1.9����ҪĿ����_______________________��
(2)������б���ʳ��ˮ��������_________________________��
(3)���������ҺA��������Ũ�����ñ�ˮԡ��ȴ�ᾧ������еIJ�����__________(���������)��
(4)������У�������ϡ�����ַ�Ӧ����������Һ��c(Cl-)=1.0mol��L-1����c(SO42-)=________[Ksp(PbSO4)=1.6��10-8]�����������ҺB�������Լ�X�������ѭ�����ã��Լ�XӦѡ�������е�_____(����)��
a.HNO3 b.Cl2 c.H2O2 d.����
(5)��Ǧ����Ǧ����ʹˮ�����ؽ���Ǧ�ĺ�����������������Ⱦ��ˮ��Һ��Ǧ�Ĵ�����̬��Ҫ��Pb2+��Pb(OH)+��Pb(OH)2��Pb(OH)3-��Pb(OH)42-������̬��ǦŨ�ȷ���x����ҺpH�仯�Ĺ�ϵ��ͼ��ʾ��

��̽��Pb2+�����ʣ���Pb2+����Һ����εμ�NaOH��Һ���ȱ���ǣ������μ�NaOH��Һ�ֱ���壬pHΪ13��14ʱ����Һ�з�������Ҫ��Ӧ�����ӷ���ʽΪ__________________��
�ڳ�ȥ��Һ�е�Pb2+������С����һ�������Լ�(DH)�������������ӣ��Ӷ�ȥ��ˮ�еĺ���Ǧ�������������ӣ�ʵ������¼���£�
���� | Pb2+ | Ca2+ | Fe3+ | Mn2+ |
����ǰŨ��/(mg��L-l) | 0.100 | 29.8 | 0.12 | 0.087 |
������Ũ��/(mg�� L-1) | 0.004 | 22.6 | 0.04 | 0.053 |
�������Լ�(DH)����Ǧ��������Ҫ�����ķ�ӦΪ��2DH(s)+Pb2+(aq)![]() D2Pb(s)+2H+(aq)������Ǧʱ����ʵ�pHԼΪ_____________����ʵ����Ǧ���ѳ���Ϊ_________________��
D2Pb(s)+2H+(aq)������Ǧʱ����ʵ�pHԼΪ_____________����ʵ����Ǧ���ѳ���Ϊ_________________��