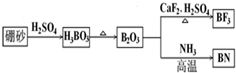
��Ŀ����
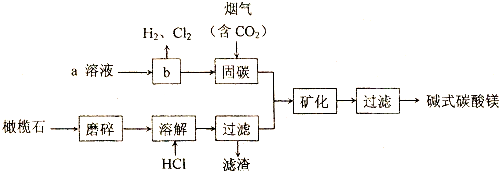
6����ú���к���SiO2��Al2O3��Fe2O3�ȣ�ijʵ���Ҷ�����д�����������ͼ��ʾ��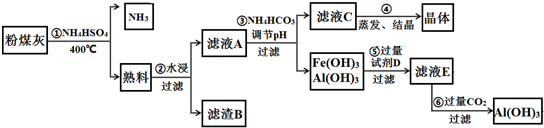
�ش��������⣺
��1���ڢٲ��õ��ġ����ϡ��п����Եijɷ���Ҫ��NH4Fe��SO4��2��NH4Al��SO4��2�ȣ�д������NH4Fe��SO4��2�Ļ�ѧ����ʽFe2O3+4NH4HSO4$\frac{\underline{\;400��\;}}{\;}$2 NH4Fe��SO4��2+2NH3��+3H2O�������ʷ�����ϵ�У�NH4Fe��SO4��2��NH4Al��SO4��2������d������ĸ����
a�������� b������ c��ǿ����� d�����ۻ�����
��2������B����Ҫ�ɷ�ΪSiO2������NaOH��Һ��Ӧ�����ӷ���ʽΪSiO2+2OH-=SiO32-+H2O��
��3����֪KSP[Fe��OH��3]=4��10-38��KSP[Al��OH��3]=1��10-33��Ϊʵ�ֲ���۵�ʵ��Ŀ�ģ�Ӧʹ��Һ��c��Fe3+����c��Al3+����С�ڻ����1��10-9 mol•L-1����Ϊ��ȫ����������ҺA����Ӧ���ڵ�pH=6����NH4HCO3����pH��ʵ��ԭ��ΪH++HCO3-=H2O+CO2���������ӷ���ʽ��ʾ����
��4��ʵ���ҽ��еڢܲ�����ʱ������������Ǿƾ��ơ�ʯ���������żܡ��������������õ��ľ�����Ҫ�ɷ��ǣ�NH4��2SO4���ѧʽ�����ڢݲ������Լ�D��NaOH��Һ���ڢ�����Al��OH��3�����ӷ���ʽΪ[Al��OH��4]-+CO2+H2O=Al��OH��3��+HCO3-��
���� ��1���������⣬Fe2O3��NH4HSO4��Ӧ���� NH4Fe��SO4��2�Ͱ�����ˮ�����ʷ�����ϵ�У�NH4Fe��SO4��2��NH4Al��SO4��2�����ڸ��Σ�����ǿ����ʣ�
��2��SiO2������һ��������Һ�������ڰ�ˮ��SiO2��NaOH��Һ��Ӧ�����ӷ���ʽΪSiO2+2OH-=SiO32-+H2O��
��3������KSP[Al��OH��3]=1��10-33��c��Al3+����С�ڻ����1��10-9 mol•L-1����Ϊ��ȫ��������ʱc��OH-��=$\root{3}{\frac{1��1{0}^{-33}}{1��1{0}^{-9}}}$=1��10-8����������Һ�У�H++HCO3-=H2O+CO2����
��4��ʵ���ҽ��еڢܲ�����ʱ����ҺC�õ�����泥�����������Ǿƾ��ơ�ʯ���������żܡ��������������ڢݲ������Լ�NaOH���������ǻ�����������ӣ��ڢ�����Al��OH��3�����ӷ���ʽΪͨ��CO2������к����ɣ�NH4��2SO4��
��� �⣺��1���������⣬Fe2O3��NH4HSO4��Ӧ���� NH4Fe��SO4��2�Ͱ�����ˮ�����ʷ�����ϵ�У�NH4Fe��SO4��2��NH4Al��SO4��2�����ڸ��Σ�����ǿ����ʣ�
�ʴ�Ϊ��Fe2O3+4NH4HSO4$\frac{\underline{\;400��\;}}{\;}$2 NH4Fe��SO4��2+2NH3��+3H2O�� d��
��2��SiO2������һ��������Һ�������ڰ�ˮ��SiO2��NaOH��Һ��Ӧ�����ӷ���ʽΪSiO2+2OH-=SiO32-+H2O��
�ʴ�Ϊ��SiO2��SiO2+2OH-=SiO32-+H2O��
��3������KSP[Al��OH��3]=1��10-33��c��Al3+����С�ڻ����1��10-9 mol•L-1����Ϊ��ȫ��������ʱc��OH-��=$\root{3}{\frac{1��1{0}^{-33}}{1��1{0}^{-9}}}$=1��10-8����������Һ�У�H++HCO3-=H2O+CO2����
�ʴ�Ϊ��6��H++HCO3-=H2O+CO2����
��4��ʵ���ҽ��еڢܲ�����ʱ����ҺC�õ�����泥�����������Ǿƾ��ơ�ʯ���������żܡ��������������ڢݲ������Լ�NaOH���������ǻ�����������ӣ��ڢ�����Al��OH��3�����ӷ���ʽΪͨ��CO2������к����ɣ�NH4��2SO4��
�ʴ�Ϊ����������������NH4��2SO4��NaOH��Һ��[Al��OH��4]-+CO2+H2O=Al��OH��3��+HCO3-��
���� ���⿼���������Ʊ�ʵ�鹤�������жϣ��������ʵ�����Ӧ�ã����ӷ���ʽ����ѧ����ʽ��д����������ˮ�����Ӧ�ã����ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

 Сѧ��ĩ���Ծ�ϵ�д�
Сѧ��ĩ���Ծ�ϵ�д�| A�� | ������ˮ�����ԣ������еμ�������ɫʯ����Һ���������Һ�ʺ�ɫ | |
| B�� | ������Һʱ������ˮ��������ƿ�̶ȣ�Ӧ�ý�ͷ�ιܽ�������Һ���� | |
| C�� | ú�Ϳ���ʯ�ͷ����ã�������ȼ�Ϻͱ������������� | |
| D�� | ����ij��Һ�Ƿ���SO42-ʱ��Ӧȡ��������Һ�����μ���BaCl2��Һ��ϡ���� |
�������ƣ�Na2FeO4����һ�����;�ˮ������ҵ���Ʊ��������Ƶķ���֮һ�ķ�Ӧԭ��Ϊ��Fe��OH��3+NaClO+NaOH��Na2FeO4+X+H2O����X�Ļ�ѧʽNaCl����Ӧ�б�������������Fe��OH��3��д��ѧʽ����
���������ϸ�ijЩ���ϻ�ϣ������Ƴɷ������ᣮ��ҵ���������������������Ҫ��Fe2O3��SiO2��Al2O3��MgO�ȣ����������Ʊ����죨Fe2O3���Ĺ������£�
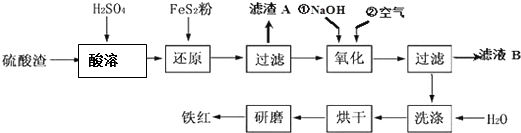
��֪����
| ������ | Fe��OH��3 | Al��OH��3 | Fe��OH��2 | Mg��OH��2 |
| ��ʼ����pH | 2.7 | 3.8 | 7.6 | 9.4 |
| ��ȫ����pH | 3.2 | 5.2 | 9.7 | 12.4 |
��1�����ܹ�����Fe2O3��ϡ���ᷴӦ�Ļ�ѧ����ʽΪFe2O3+3H2SO4�TFe2��SO4�� 3+3H2O��������A����Ҫ�ɷݵĻ�ѧʽΪSiO2��
��2����ԭ�����м���FeS2��Ŀ���ǽ���Һ�е�Fe3+��ԭΪFe2+��������������ΪH2SO4��д���÷�Ӧ�����ӷ���ʽ��FeS2+14 Fe3++8 H2O=15Fe2++2 SO42-+16 H+��
��3��Ϊ��ȷ������������ʹ��ȣ����������м�NaOH������Һ��pH�ķ�Χ��3.2��3.8�������NaOH������Һ��pH=a������ҺB�е�c��Fe3+��=4��10��4-3a����25��ʱ��Ksp[Fe��OH��3]=4��10-38��
��4����ҺB�п��Ի��յ�������Na2SO4��Al2��SO4��3��MgSO4��
 C��g��+3D��g������H��0���ֽ�2 mol A��2 mol B�������ΪV�ļ���������2 mol C��6 mol D������������ʹ�������ڷ�Ӧ��ʼǰ�����Ϊ2V����ͼ1����
C��g��+3D��g������H��0���ֽ�2 mol A��2 mol B�������ΪV�ļ���������2 mol C��6 mol D������������ʹ�������ڷ�Ӧ��ʼǰ�����Ϊ2V����ͼ1����
 2NH3��g�� ��H<0����lL�ܱ������м���0.1 mol N2��0.3mol H2��ʵ��١��ڡ�����c��N2����ʱ�䣨t���ı仯����ͼ��ʾ��
2NH3��g�� ��H<0����lL�ܱ������м���0.1 mol N2��0.3mol H2��ʵ��١��ڡ�����c��N2����ʱ�䣨t���ı仯����ͼ��ʾ��
 �е�______________��_____________������ĸ��ţ���
�е�______________��_____________������ĸ��ţ��� N2O4��g����
N2O4��g����

 ����غ����������Ʒ���������ԭ��Ӧ����Cl��-1�ۣ���S��+6�ۣ���������ͼ��ʾ����֪�����Ӧ����������Һ��c��H+��������ӿ죮
����غ����������Ʒ���������ԭ��Ӧ����Cl��-1�ۣ���S��+6�ۣ���������ͼ��ʾ����֪�����Ӧ����������Һ��c��H+��������ӿ죮