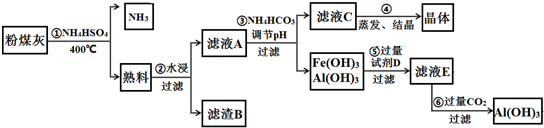
��Ŀ����
һ���¶��£��п��淴Ӧ��2A��g��+2B��g�� C��g��+3D��g������H��0���ֽ�2 mol A��2 mol B�������ΪV�ļ���������2 mol C��6 mol D������������ʹ�������ڷ�Ӧ��ʼǰ�����Ϊ2V����ͼ1����
C��g��+3D��g������H��0���ֽ�2 mol A��2 mol B�������ΪV�ļ���������2 mol C��6 mol D������������ʹ�������ڷ�Ӧ��ʼǰ�����Ϊ2V����ͼ1����
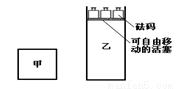
�����������з�Ӧ��˵����ȷ����
A����������ƽ���������ͬ���¶�ʱ���������ķ�Ӧ���ʴ���������������
B���������еķ�Ӧ����ƽ��ʱ��ƽ�������и���ݵ�����ٷ������ͬ�����������ܶȲ�ͬ
C���������еķ�Ӧ�ȴﵽ��ѧƽ��״̬
D���ڼ��������ٳ���2mol A��2 mol B��ƽ����������C�����ʵ�������������C�����ʵ�����2��
��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
7�����й���16O��18O��˵����ȷ���ǣ�������
| A�� | 16O��18O��Ϊͬλ�� | B�� | 16O��18O���в�ͬ�ĵ����� | ||
| C�� | 16O��18O��Ϊͬ�������� | D�� | 16O��18O��ѧ���ʲ�ͬ |
7���±����й����ӷ���ʽ�����ۺ������ǣ�������
| ѡ�� | ��ѧ��Ӧ�����ӷ���ʽ | ���� |
| A | AlCl3��Һ�м��������ˮ�� Al3++3NH3•H2O�TAl��OH��3��+3NH4+ | ������Ԫ�صIJ���Ӧ����AlO2- |
| B | ��������ͨ���廯������Һ�У� 3Cl2+2Fe2++4Br-�T6Cl-+2Fe3++2Br2 | ��ȷ |
| C | �ð�ˮ���չ����������� 2NH3•H2O+SO2�T2NH4++SO32-ʮH2O | ��ȷ |
| D | �Ȼ������ˮ�� NH4++2H2O�TH3O++NH3•H2O | �����Ȼ���ܽ���ˮ�������仯������д���ӷ���ʽ |
| A�� | A | B�� | B | C�� | C | D�� | D |


 2NH3(g) ��H����92.4 kJ��mol��1��һ�ֹ�ҵ�ϳɰ��ļ�ʽ����ͼ���£�
2NH3(g) ��H����92.4 kJ��mol��1��һ�ֹ�ҵ�ϳɰ��ļ�ʽ����ͼ���£�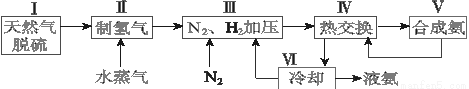
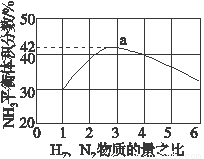
 �ȡ����������к��ȵ���ֵ��______________�����ȡ��� ������ȡ�����
�ȡ����������к��ȵ���ֵ��______________�����ȡ��� ������ȡ�����
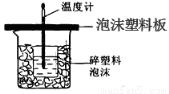
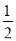 O2��g����CO��g������H1 C��s����O2��g����CO2��g������H2
O2��g����CO��g������H1 C��s����O2��g����CO2��g������H2 ��ͨ������ȷ����Ӧ�������ɣ��û�ѧʽ��ʾ���� ������Ӧ��������g��Ϊ ��
��ͨ������ȷ����Ӧ�������ɣ��û�ѧʽ��ʾ���� ������Ӧ��������g��Ϊ ��