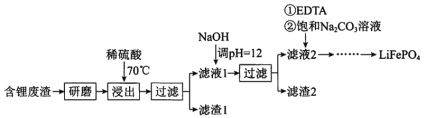
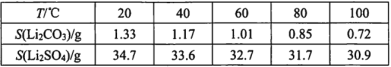
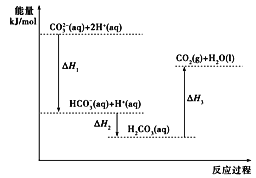
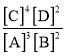��Ŀ����
����Ŀ����ͼ�ǽ���þ��±�ص���(X2)��Ӧ�������仯ʾ��ͼ������˵����ȷ����( )
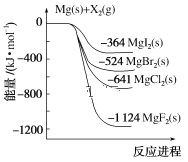
A.��MgCl2��ȡMg�Ƿ��ȹ���
B.���ȶ��ԣ�MgI2��MgBr2��MgCl2��MgF2
C.����þ��±�ص���(X2)�ķ�Ӧ���Ƿ��ȷ�Ӧ
D.��ͼ��֪���¶���MgBr2(s)��Cl2(g)��Ӧ���Ȼ�ѧ����ʽΪMgBr2(s)��Cl2(g)===MgCl2(s)��Br2(g)����H����117 kJ��mol��1
���𰸡�C
��������
A. ��ͼ���Կ�������Mg����MgCl2�Ƿ��ȹ��̣�����MgCl2��ȡMg�����ȹ��̣�A����
B. ���������е�����Խ�ͣ�����Խ�����ȶ���Խǿ����ͼ��֪������MgI2��MgBr2��MgCl2��MgF2���������ȶ��ԣ�MgI2<MgBr2<MgCl2<MgF2��B����
C. ��Ϊ��Ӧ�������������������������������Խ���þ��±�ص���(X2)�ķ�Ӧ���Ƿ��ȷ�Ӧ��C��ȷ��
D. ��ͼ��֪���¶���MgBr2(s)��Cl2(g)��Ӧ���Ȼ�ѧ����ʽΪMgBr2(s)��Cl2(g)=MgCl2(s)��Br2(g)����H��-(641-524) kJ��mol��1=-117 kJ��mol��1��D����
��ѡC��

����Ŀ���������ػ������ڻ�����ҽҩ�����ϵ��������Ź㷺��Ӧ�á��ش��������⣺
��1�������Ǿ����۷��ӣ����ͽṹ�����з�����֤�������ǷǾ������_____��
A������ B��ԭ�ӷ������ C���˴Ź����� D��X�������䷨
��2����̬Asԭ�ӵĺ�������Ų�ʽΪ_____��Asԭ�ӵ��������������£�
��һ������ | �ڶ������� | ���������� | ���ĵ����� | ��������� | ���������� |
947.0 | 1798 | 2735 | 4837 | 6043 | 12310 |
Ϊʲô�����������������������ϴ�_____��
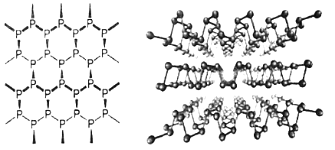
��3�����������Ͷ�ά�뵼����ϣ�����Ƭ��ṹ������ͼ��ʾ����Pԭ�ӵ��ӻ���ʽΪ_____�������֮��������Ϊ_____��
��4��GaAs���۵�Ϊ1238�棬������״̬�����磬�侧���ṹ����ͼ��ʾ���þ��������Ϊ_____��ÿ��Asԭ����Χ�����Asԭ����ĿΪ_____����ͬһ��Gaԭ�Ӿ��������Asԭ�ӹ��ɵĿռ乹��Ϊ_____��һ��GaAs��������λ������ĿΪ_____��
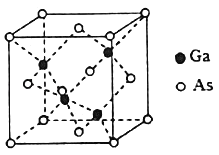
��5����֪GaAs���ܶ�Ϊdg/cm3��Ħ������ΪMg/mol�������ӵ�������NA��ʾ�����������As��Gaԭ�Ӻ˼��Ϊ_____ nm����ʽ��ʾ����
����Ŀ����ҵ�ϳɰ��ķ�ӦΪN2(g)��3H2(g)![]() 2NH3(g)����֪���л�ѧ���ļ��ܣ�
2NH3(g)����֪���л�ѧ���ļ��ܣ�
��ѧ�� | ����kJ/mol |
H-H | 436 |
N-H | 391 |
N | 946 |
����˵����ȷ����
A. �÷�ӦΪ���ȷ�ӦB. �÷�Ӧ�з�Ӧ��������������������������
C. ��Ӧ����H��92 kJ��mol��1D. ����1 mol NH3�ų�92 kJ����