��Ŀ����
��9�֣�ijѧ����0.2000mol��L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ������������£�
����ȡ20.00mL����Һע��ྻ����ƿ�У�������3�η�̪��Һ��
���ñ�Һ�ζ����յ㣬��¼�ζ���Һ������������������±���
��ش��������⣺
��1��������У���ȡ20.00mL����ҺӦʹ��________________(����������)��������ƿװҺǰ������������ˮ����ʹ�ⶨ���_______________���ƫ����ƫС������Ӱ�족����
��2��������У��ζ�ʱ�۾�Ӧע��________________�����������ƣ����жϵ���ζ��յ��������___________________________________________��
��3����һ�εζ���¼��NaOH��Һ��������Զ��ں����ε����������ܵ�ԭ����_______________________������ĸ��
A���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����ʱ������
B����ƿװҺǰ�ô���Һ��ϴ
C��NaOH��Һ����ʱ��������в���Na2CO3����
D���ζ��յ�ʱ�����Ӷ���
��4�������ϱ���¼���ݣ�ͨ������ɵø������Ũ��Ϊ______ mol��L-1 ��
����ȡ20.00mL����Һע��ྻ����ƿ�У�������3�η�̪��Һ��
���ñ�Һ�ζ����յ㣬��¼�ζ���Һ������������������±���
| �ζ����� | ���� ��� ��� | NaOH��Һ���������mL�� | |
| �ζ�ǰ | �ζ��� | ||
| 1 | 20.00 | 0.00 | 18.10 |
| 2 | 20.00 | 0.00 | 16.30 |
| 3 | 20.00 | 0.00 | 16.22 |
��1��������У���ȡ20.00mL����ҺӦʹ��________________(����������)��������ƿװҺǰ������������ˮ����ʹ�ⶨ���_______________���ƫ����ƫС������Ӱ�족����
��2��������У��ζ�ʱ�۾�Ӧע��________________�����������ƣ����жϵ���ζ��յ��������___________________________________________��
��3����һ�εζ���¼��NaOH��Һ��������Զ��ں����ε����������ܵ�ԭ����_______________________������ĸ��
A���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����ʱ������
B����ƿװҺǰ�ô���Һ��ϴ
C��NaOH��Һ����ʱ��������в���Na2CO3����
D���ζ��յ�ʱ�����Ӷ���
��4�������ϱ���¼���ݣ�ͨ������ɵø������Ũ��Ϊ______ mol��L-1 ��
��9�֣�
��1����ʽ�ζ��ܣ�1�֣�����Ӱ�죨1�֣�
��2����ƿ ��1�֣���ƿ����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ��2�֣�
��1�֣���ƿ����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ��2�֣�
��3��AB(2��)
��4��0.1626(2��)
��1����ʽ�ζ��ܣ�1�֣�����Ӱ�죨1�֣�
��2����ƿ
 ��1�֣���ƿ����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ��2�֣�
��1�֣���ƿ����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ��2�֣���3��AB(2��)
��4��0.1626(2��)
��

��ϰ��ϵ�д�
 �����ߴ���ϵ�д�
�����ߴ���ϵ�д�
�����Ŀ
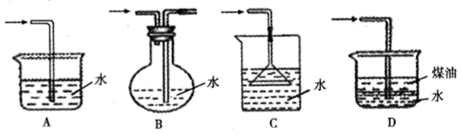
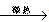 NaHSO4��HNO3������ȡHNO3ʱ����ͼ����װ�������ʺϵ���
NaHSO4��HNO3������ȡHNO3ʱ����ͼ����װ�������ʺϵ���



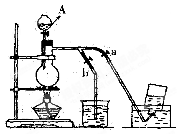
 �������β���a��bֹˮ�У���ͬ����
�������β���a��bֹˮ�У���ͬ����

 mL D.200mL
mL D.200mL �� ��
�� �� ˮ���̶ȴ�
ˮ���̶ȴ�