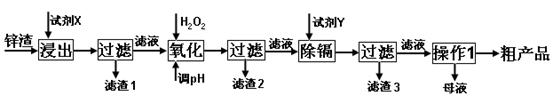
��Ŀ����
(2��)����ʵ��������ʵ����ʵ����������ȷ��˵���� ��
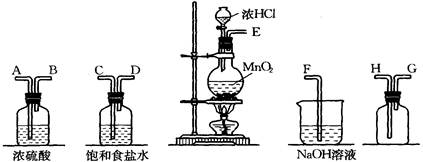
���������Ũ��Һմ��Ƥ���ϣ�Ӧ�����þƾ�ϴ��
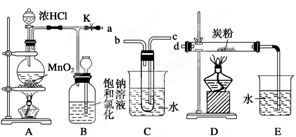
�ڳ�ȥ�����л��е�������ϩ�������û����ͨ��ʢ������KMnO4��Һ��ϴ��װ��
��ʵ����������������Ũ���ᡢŨ����Ļ�����е��뱽����50��60���ˮԡ�м���
��ʵ������ȡ��ϩʱ������ˮ�Ҵ���10mol��L-1 H2SO4��ϼ��ȵ�1700C�����Ƶ���ϩ
��ֻ����ˮ�Ϳɽ��������Ȼ�̼���Ҵ�����ϩ����Һ��������
����±�����е�±���ӣ��ǽ����������Թܣ�Ȼ�����NaOH��У��漴�������������ɡ�
���������Ũ��Һմ��Ƥ���ϣ�Ӧ�����þƾ�ϴ��
�ڳ�ȥ�����л��е�������ϩ�������û����ͨ��ʢ������KMnO4��Һ��ϴ��װ��
��ʵ����������������Ũ���ᡢŨ����Ļ�����е��뱽����50��60���ˮԡ�м���
��ʵ������ȡ��ϩʱ������ˮ�Ҵ���10mol��L-1 H2SO4��ϼ��ȵ�1700C�����Ƶ���ϩ
��ֻ����ˮ�Ϳɽ��������Ȼ�̼���Ҵ�����ϩ����Һ��������
����±�����е�±���ӣ��ǽ����������Թܣ�Ȼ�����NaOH��У��漴�������������ɡ�
��2�֣��٢ۢ�
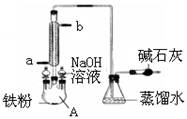
�ڴ�����ȥ�����л��е�������ϩ�������û����ͨ��ʢ����ˮ��Һ��ϴ��װ��
�ܴ���ʵ������ȡ��ϩʱ������ˮ�Ҵ���18.4mol��L-1 H2SO4��ϼ��ȵ�1700C�����Ƶ���ϩ
��������±�����е�±���ӣ��ǽ����������Թܣ�Ȼ�����NaOH��У��ټ��������ϡ�����������漴�������������ɡ�
�ܴ���ʵ������ȡ��ϩʱ������ˮ�Ҵ���18.4mol��L-1 H2SO4��ϼ��ȵ�1700C�����Ƶ���ϩ
��������±�����е�±���ӣ��ǽ����������Թܣ�Ȼ�����NaOH��У��ټ��������ϡ�����������漴�������������ɡ�

��ϰ��ϵ�д�
 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�
�����Ŀ
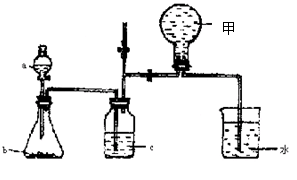

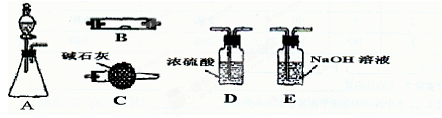
 ���
���