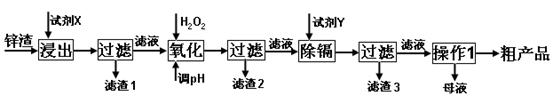
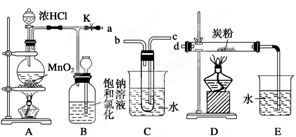
��Ŀ����
��12�֣���ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص�Ũ�����Լ���ǩ�ϵIJ������ݡ�

���ø�Ũ��������100 mL 1 mol������1��ϡ���ᡣ�ɹ�ѡ�õ������У��ٽ�ͷ�ιܣ����� ƿ�����ձ����� ҩ�ף�����Ͳ����������ƽ����ش��������⣺
(1)ʢ��Ũ������Լ�ƿ��ǩ��Ӧӡ�����о�ʾ����е� ��

(2)����ϡ����ʱ����ȱ�ٵ����� �� (д��������)
(3)�����㣬����100 mL1 mol������1��ϡ������Ҫ����Ͳ��ȡ����Ũ��������Ϊ �� mL����ȡ����ʱӦѡ�� �� mL������Ͳ������ĸ���ţ�
A.10 mL B.50 mL C.100 mL D.200mL
mL D.200mL
(4)���ձ���ϡ��Ũ�����ʵ�����Ϊ �� ; ����ϡ�����У�����Ũ�����մ�����ϣ���������Ϊ �� ��
�� ��
(5)�������Ƶ�ϡ������вⶨ��������Ũ�ȴ���1 mol������1�����ƹ��������и��������������������� �� ��
A.����Ͳ��ȡŨ����ʱ�����ӿ̶���ȡŨ����
B.����ƿ������ˮϴ�Ӻ�δ���������������ˮ
C.��ϡ�ͺ��ϡ��������ת������ƿ�����žͽ����Ժ��ʵ�����
D.ת����Һʱ��������������Һ��������ƿ����
E.����ʱ����������ƿ�̶��߽��ж���
F.���ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ��㲹�伸�� ˮ���̶ȴ�
ˮ���̶ȴ�

���ø�Ũ��������100 mL 1 mol������1��ϡ���ᡣ�ɹ�ѡ�õ������У��ٽ�ͷ�ιܣ����� ƿ�����ձ����� ҩ�ף�����Ͳ����������ƽ����ش��������⣺
(1)ʢ��Ũ������Լ�ƿ��ǩ��Ӧӡ�����о�ʾ����е� ��

(2)����ϡ����ʱ����ȱ�ٵ����� �� (д��������)
(3)�����㣬����100 mL1 mol������1��ϡ������Ҫ����Ͳ��ȡ����Ũ��������Ϊ �� mL����ȡ����ʱӦѡ�� �� mL������Ͳ������ĸ���ţ�
A.10 mL B.50 mL C.100
 mL D.200mL
mL D.200mL(4)���ձ���ϡ��Ũ�����ʵ�����Ϊ �� ; ����ϡ�����У�����Ũ�����մ�����ϣ���������Ϊ
 �� ��
�� ��(5)�������Ƶ�ϡ������вⶨ��������Ũ�ȴ���1 mol������1�����ƹ��������и��������������������� �� ��
A.����Ͳ��ȡŨ����ʱ�����ӿ̶���ȡŨ����
B.����ƿ������ˮϴ�Ӻ�δ���������������ˮ
C.��ϡ�ͺ��ϡ��������ת������ƿ�����žͽ����Ժ��ʵ�����
D.ת����Һʱ��������������Һ��������ƿ����
E.����ʱ����������ƿ�̶��߽��ж���
F.���ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ��㲹�伸��
 ˮ���̶ȴ�
ˮ���̶ȴ�
��

��ϰ��ϵ�д�
�����Ŀ
 ���
��� %��Ũ����(��= 1.84g/cm3)����Ũ��Ϊ0.5 mol/L��ϡ����500mL�������¼�������,�밴Ҫ��ش���������:
%��Ũ����(��= 1.84g/cm3)����Ũ��Ϊ0.5 mol/L��ϡ����500mL�������¼�������,�밴Ҫ��ش���������:  ��
��
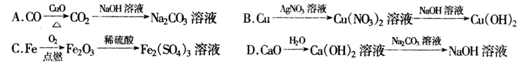
 LH2SO4������400mLˮ
LH2SO4������400mLˮ 5mol/LH2SO4����500mLˮϡ��
5mol/LH2SO4����500mLˮϡ��