��Ŀ����
12��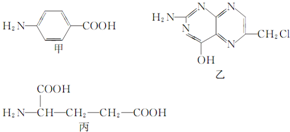 Ҷ����ά����B��֮һ�����������мס��ҡ����������ʺϳɣ�
Ҷ����ά����B��֮һ�����������мס��ҡ����������ʺϳɣ���1�����к������������Ȼ��������ƣ���
��2�����й����ҵ�˵����ȷ����ac������ţ���
a��������̼ԭ���뵪ԭ�ӵĸ�������7��5
b�����ڷ����廯����
c����������������������������Һ��Ӧ
d�����ڱ��ӵ�ͬϵ��
��3�����DZ���ͬ���칹�壬�����������������������Ľṹ��ʽΪ
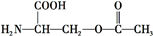 ��
��a������
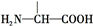
b����ϡ������ˮ������������
��4��д����������������Һ��ˮ��Ļ�ѧ����ʽ
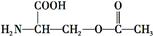 +2NaOH��HOCH2CH��NH2��COONa+H2O+CH3COONa��
+2NaOH��HOCH2CH��NH2��COONa+H2O+CH3COONa��
���� ��1�����а������Ȼ���
��2��a���÷�����C��Nԭ�Ӹ����ֱ���7��5��
b�����б������л������ڷ����廯���
c�������ܺ��ᷴӦ����ԭ���ܺͼ���Һ��Ӧ��
d���÷����к���Nԭ�ӣ�
��3������ͬ���칹�嶡�к���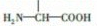 ����ϡ������ˮ�����������ɣ�˵����������������Ϊ����ij�����ݴ��ж϶��Ľṹ��ʽ��
����ϡ������ˮ�����������ɣ�˵����������������Ϊ����ij�����ݴ��ж϶��Ľṹ��ʽ��
��4����Ϊ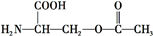 ���������������ڼ���������ˮ�⣬���Ȼ����������Ʒ����кͷ�Ӧ��
���������������ڼ���������ˮ�⣬���Ȼ����������Ʒ����кͷ�Ӧ��
��� �⣺��1�����а������Ȼ�������������Ϊ�Ȼ����ʴ�Ϊ���Ȼ���
��2��a���÷�����C��Nԭ�Ӹ����ֱ���7��5�����Է�����̼ԭ���뵪ԭ�ӵĸ�������7��5������ȷ��
b�����б������л������ڷ����廯����÷�����û�б��������Բ����ڷ����廯����ʴ���
c�������ܺ��ᷴӦ����ԭ���ܺͼ���Һ��Ӧ�����Ը����ʼ�������������������������Һ��Ӧ������ȷ��
d���÷����к���Nԭ�ӣ������ڱ��ӵ�ͬϵ��ʴ���
�ʴ�Ϊ��ac��
��3������ͬ���칹�嶡�к���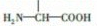 ����ϡ������ˮ�����������ɣ�˵����������������Ϊ����ij�������Զ��Ľṹ��ʽΪ
����ϡ������ˮ�����������ɣ�˵����������������Ϊ����ij�������Զ��Ľṹ��ʽΪ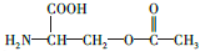 ��
��
�ʴ�Ϊ��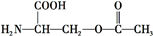 ��
��
��4����Ϊ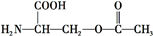 ���������������ڼ���������ˮ�⣬���Ȼ����������Ʒ����кͷ�Ӧ������ʽΪ
���������������ڼ���������ˮ�⣬���Ȼ����������Ʒ����кͷ�Ӧ������ʽΪ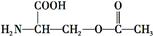 +2NaOH��HOCH2CH��NH2��COONa+H2O+CH3COONa��
+2NaOH��HOCH2CH��NH2��COONa+H2O+CH3COONa��
�ʴ�Ϊ��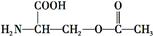 +2NaOH��HOCH2CH��NH2��COONa+H2O+CH3COONa��
+2NaOH��HOCH2CH��NH2��COONa+H2O+CH3COONa��
���� ���⿼�����л���Ľṹ�����ʣ��漰�л���ϳɡ���Ӧ���͵��жϡ����������֪ʶ�㣬�������ʷ�Ӧ�ص�ȷ����Ӧ���͡���������ͼ�з�Ӧ�P��Ӧ����ȷ��������ٽ�ϻ�����������Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ������Һ��pH=a��������Һϡ��1������Һ��pH=b����a��b | |
| B�� | pH=12��NaOH��Һ��pH=2��CH3COOH��Һ�������ϣ���Ϻ���ҺpH��7 | |
| C�� | ���ʵ���Ũ�Ⱦ�Ϊ0.01 mol•L-1��CH3COOH��CH3COONa��Һ�������ϣ�c��CH3COOH��+c��CH3COO-��=0.01mol•L-1 | |
| D�� | ��ʹʯ����Һ������Һ�У�Fe2+��Na+��NO3-��Cl-�ܴ������� |
| A�� | ��ˮ�ܵ��磬˵�������ǵ���� | |
| B�� | ���Ƶ���ˮ�д���3 �ַ��ӣ�4 ������ | |
| C�� | ˫��ˮ����Ϊ����ɫ���������仹ԭ����ΪO2 | |
| D�� | ��ˮ��Ũ�����Ũ���ᰴ�����1��3 ��� |
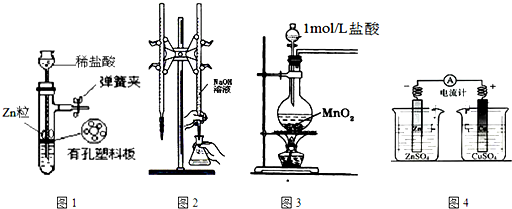
| A�� | ��ͼ1��ʾװ����ȡ����H2 | |
| B�� | ��ͼ2��ʾװ���ñ�Ũ�ȵ�����������Һ�ⶨ�����Ũ�� | |
| C�� | ��ͼ3��ʾװ����ȡ����Cl2 | |
| D�� | ��ͼ4��ʾװ��ȷ���������е���ͨ������ȷ���������� |
| ת�� | ���� | |
| A | CuS+H2SO4=CuSO4+H2S�� | ���ԣ�H2SO4��H2S |
| B | AgCl��s��+I-��aq��?AgI��s��+Cl-��aq�� | Kap��AgCl����Kap��AgI�� |
| C | 2Fe+3Cl2$\frac{\underline{\;��ȼ\;}}{\;}$2FeCl3��Fe+S$\frac{\underline{\;����\;}}{\;}$FeS | �����ԣ�Cl2��S |
| D | C��s��ʯī��=C��s�����ʯ����H=+1.9kL/mol | �ȶ��ԣ�ʯī�����ʯ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | �ó��������������������������仯 | |
| B�� | ����ˮ��þ���Ͻ�ըʱ������ | |
| C�� | �ƹ�ʹ��úҺ�������Ƽ����������������̼���ŷ� | |
| D�� | ��������K3C60������״̬���ܹ����磬˵��K3C60�ǵ���� |
2NH3��g��+CO2��g��?CO��NH2��2��I��+H2O��I����H��I��
��1����֪�ϳ����صķ�Ӧ���������У�
2NH3��g��+CO2��g��?NH2COONH4��s����H1
NH2COONH4��s��?CO��NH2��2��I��+H2O��I����H2
�������仯������ͼ1��ʾ�����H����H1�͡�H2�ɴ�С��˳��Ϊ��H2����H����H1��
��2����һ����պ����ܱ������г���CO2��NH3������Ӧ��I���ϳ����أ��㶨�¶��»��������NH3�����������ͼ2��ʾ��
A�������Ӧ����v����CO2����B����淴Ӧ����v����CO2�������������������=������CO2��ƽ��ת����Ϊ75%��
��3����һ�����İ�������粒������ں�����������У�������Ӧ��H2NCOONH4��s��?2NH3��g��+CO2��g�����ڲ�ͬ�¶ȣ�T1��T2���£��÷�Ӧ��ƽ��״̬ʱ�������ݼ��±���
| �¶� | ƽ��Ũ��/��mol•L-1�� | |
| c��NH3�� | c��CO2�� | |
| T1 | 0.1 | |
| T2 | 0.1 | |
��������˵���÷ֽⷴӦ�ﵽƽ��״̬����ac������ţ���
a��v������NH3��=2v������CO2��
b���ܱ����������ʵ�����������
c���ܱ������л��������ܶȲ���
d���ܱ������а����������������
��4����������識���ˮ���̼��泥�����������ˮ������ף�25��ʱ����1L 0.1mol•L-1�����������백������立�ĩ����Һ�����ԣ�������Һ����仯��������ȥ0.052mol��������泥���ʱ��Һ�м�������̼Ԫ�أ���ʱ��Һ��c��NH4+��=0.1mol/L��NH4+ˮ��ƽ�ⳣ��ֵΪ4��10-9��

 FeCl3���о�ˮ���ã�����ʴ�豸�����ۺ��Ȼ�����һ�����͵���������������ˮ��FeCl3��Ч���Ҹ�ʴ��С����ش��������⣺
FeCl3���о�ˮ���ã�����ʴ�豸�����ۺ��Ȼ�����һ�����͵���������������ˮ��FeCl3��Ч���Ҹ�ʴ��С����ش��������⣺ ʵ�����Ա���ȩΪԭ���Ʊ����屽��ȩ��ʵ��װ�ü�ͼ��������ʵķе������������ʵ�鲽��Ϊ��
ʵ�����Ա���ȩΪԭ���Ʊ����屽��ȩ��ʵ��װ�ü�ͼ��������ʵķе������������ʵ�鲽��Ϊ��