��Ŀ����
1��5��3�գ��㶫ʡ���ֲ��ֻ���ʹ������������ڶ���ҩ����˾�����ġ���������ע��Һ�����������ز�����Ӧ�����ж���������Ŀǰ�����ҩ���ij����Ѿ�ˮ��ʯ�����ù�˾�ɹ���Ա�ڹ���ҩ�ø��ϱ��������ṹ��ʽΪHOCH2CH2CH2OH��ʱ��Ϊͼ���ˣ������˼�ð�������Ķ��ʴ����ṹ��ʽΪHOCH2CH2-O-CH2CH2OH������Ϊ�������ڡ���������ע��Һ�����������Ӷ���ɶ������˵IJҾ磮���ڱ���������ʴ���˵������ȷ���ǣ�������| A�� | ����������ʴ���Ϊͬϵ�� | |
| B�� | �������Ͷ��ʴ�����Ϊͬ���칹�� | |
| C�� | 1 mol����������ʴ��Ļ������������������Ʒ�Ӧ����22.4 L���� | |
| D�� | ����������ʴ����ܷ���ȡ����Ӧ |
���� A������������ʴ����еĹ����Ų���ȫ��ͬ��
B���������Ͷ��ʴ�����ʽ����ͬ��
C���������Ͷ��ʴ����ǻ���Na��Ӧ�������������ɵ�������һ�����ڱ���£�
D���������Ͷ��ʴ������д��ǻ������Է���ȡ����Ӧ��
��� �⣺�⣺A������������ʴ����еĹ����Ų���ȫ��ͬ�����߲���ͬϵ���A����
B���������Ͷ��ʴ�����ʽ����ͬ�����߲���ͬ���칹�壬��B����
C.1mol��������1mol���ʴ�������2mol�ǻ���1 mol����������ʴ��Ļ������������������Ʒ�Ӧ����1mol������������һ�����ڱ���£�����������һ��Ϊ22.4L����C����
D���������Ͷ��ʴ������д��ǻ������д������ʣ����Է���ȡ����Ӧ����D��ȷ��
��ѡD��
���� ���⿼���л���Ľṹ�����ʡ�ͬϵ�ͬ���칹�壬Cѡ��Ϊ�״��㣬ѧ������������������״̬��

��ϰ��ϵ�д�
 ������ʱͬ����ϰ��ϵ�д�
������ʱͬ����ϰ��ϵ�д�
�����Ŀ
9���뵼�幤ҵ��ʯӢɰ��ԭ��ͨ������������Ҫ��Ӧ�������裺
Si��s����ʯӢɰ��+2C��s���TSi��s�����ֹ裩+2CO��g����H=+682.44kJ/mol
Si��s�����ֹ裩+2Cl2��s���TSiCl4��g������H=-657.01kJ/mol
SiCl4��g��+2Mg��s���T2MgCl2��s��+Si��s�������裩����H=-625.63kJ/mol
������56.00g����ķ�Ӧ��Ϊ��������
Si��s����ʯӢɰ��+2C��s���TSi��s�����ֹ裩+2CO��g����H=+682.44kJ/mol
Si��s�����ֹ裩+2Cl2��s���TSiCl4��g������H=-657.01kJ/mol
SiCl4��g��+2Mg��s���T2MgCl2��s��+Si��s�������裩����H=-625.63kJ/mol
������56.00g����ķ�Ӧ��Ϊ��������
| A�� | 600.20 kJ•mol-1 | B�� | +1200.40 kJ•mol-1 | ||
| C�� | -1965.08 kJ•mol-1 | D�� | -1200.40 kJ•mol-1 |
6�����������϶���ȷ���ǣ�������
| A�� | �����Ӿ����в����ܴ��ڷǼ��Լ� | |
| B�� | �ڹ��ۻ�����ķ��Ӿ����в����ܴ������Ӽ� | |
| C�� | �ڼ��Է����в����ܴ��ڷǼ��Լ� | |
| D�� | ��ԭ�Ӿ����в����ܴ��ڼ��Թ��ۼ� |
13��PH3��좣����ӿռ乹���������Σ����¹���PH3�������У���ȷ���ǣ�������
| A�� | PH3�ǷǼ��Է��� | B�� | PH3�����е�P-H ���ǷǼ��Լ� | ||
| C�� | PH3����ԭ���ӻ��������Ϊsp2�� | D�� | PH3��������δ�ɼ��ĵ��Ӷ� |
10����ǿ������Һ��һ���ܴ���������������ǣ�������
| A�� | OH-��Na+��K+��HCO3- | B�� | Na+��K+��Cl-��Ag+ | ||
| C�� | Mg+��SO42-��Na+��NO3- | D�� | MnO4-��Fe2+��CO32-��Ba2+ |
11���谢���ӵ�����ΪNA������˵����ȷ���ǣ�������
| A�� | ���³�ѹ�£�2g��������ԭ������0.5NA | |
| B�� | ���³�ѹ�£�11.2L����������������0.5NA | |
| C�� | ͬ��ͬѹ�£��ܶ���ͬ�����������Ħ��������ͬ | |
| D�� | ͬ��ͬѹ�£�ԭ��������NA����������������ͬ |
 X��Y��Z�����ֶ�����Ԫ�أ�X��Z��������֮����Y����������ȣ�Z�ĵ��Ӳ�����X�ĵ��Ӳ�����2����A��B��C��D��E��F����ѧ��ѧ�еij������ʣ���������������Ԫ���е�һ�֡����ֻ�������ɣ�����A����ʹʪ���ɫʯ����ֽ���������壬D��E���� ���ᣬF��һ�ֵ��ʣ���Ӧ�ܾۢ������������½��У���ת����ϵ��ͼ��ʾ��
X��Y��Z�����ֶ�����Ԫ�أ�X��Z��������֮����Y����������ȣ�Z�ĵ��Ӳ�����X�ĵ��Ӳ�����2����A��B��C��D��E��F����ѧ��ѧ�еij������ʣ���������������Ԫ���е�һ�֡����ֻ�������ɣ�����A����ʹʪ���ɫʯ����ֽ���������壬D��E���� ���ᣬF��һ�ֵ��ʣ���Ӧ�ܾۢ������������½��У���ת����ϵ��ͼ��ʾ��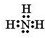
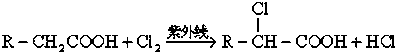 ��
��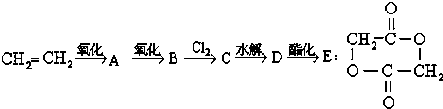
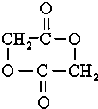 +2H2O��
+2H2O��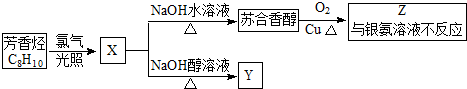
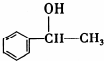 �������ܷ������л���Ӧ�����Тܡ��ޣ�
�������ܷ������л���Ӧ�����Тܡ��ޣ�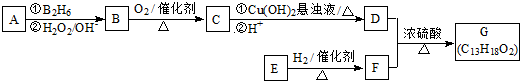
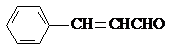 ��
��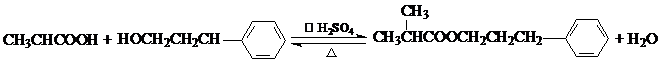 ��
��