��Ŀ����
16�������ǹ������⻯���ҵ���ð����ʹ��������ͭ{[Cu��NH3��2]Ac}�Ļ��Һ������һ����̼�������CH3COO-��дΪAc-������Ӧ����ʽΪ��[Cu��NH3��2]Ac+CO+NH3?[Cu��NH3��3CO]Ac����д����̬Cuԭ�ӵĵ����Ų�ʽ[Ar]3d104s1��
�ڰ�ˮ��Һ�и�Ԫ��ԭ�ӵĵ縺�ԴӴ�С����˳��ΪO��N��H��
�۴�������е�����̼ԭ�ӣ�����-CH3��̼���Ȼ���-COOH��̼���ӻ���ʽ�ֱ���sp3��sp2��
��������[Cu��NH3��3CO]Ac��������ѧ��������abcd��
a�����Ӽ� b����λ�� c���Ҽ� d����
���� �ٸ���Ԫ�ط��ţ��ж�Ԫ��ԭ�ӵĺ�����������ٸ��ݺ�������Ų�������д��
�ڰ�ˮ��Һ�к�N��O��H����Ԫ�أ���ĵ縺����С����ͬ����������ҵ縺�����ݴ��жϣ�
�۴�������е�����̼ԭ�ӣ�����-CH3��̼����sp2�ӻ����Ȼ���-COOH��̼����sp2�ӻ���
�ܸ���[Cu��NH3��3CO]Ac�Ľṹ�жϴ��ڵĻ�ѧ�����ͣ�
��� �⣺��1����CuԪ��Ϊ29��Ԫ�أ�ԭ�Ӻ�����29�����ӣ����Ժ�������Ų�ʽΪ��[Ar]3d104s1���ʴ�Ϊ��[Ar]3d104s1��
�ڰ�ˮ��Һ�к�N��O��H����Ԫ�أ���ĵ縺����С����ͬ����������ҵ縺�����ʵ縺�ԴӴ�С������˳��ΪO��N��H���ʴ�Ϊ��O��N��H��
�۴�������е�����̼ԭ�ӣ�����-CH3��̼����sp3�ӻ����Ȼ���-COOH��̼����sp2�ӻ����ʴ�Ϊ��sp3��sp2��
��Cu2+��NH3������λ����NH3��N��H�γɦҼ���[Cu��NH3��3CO]+��Ac-֮�������Ӽ���Ac-����̼��˫�������Ի��Цм��������ڵĻ�ѧ������Ϊ����λ�����Ҽ����м������Ӽ�����ѡabcd��
���� ���⿼���������Ų�ʽ���縺�ԡ��ӻ����͵��жϡ���ѧ����֪ʶ���Ƕ���ѧ֪ʶ���ۺϿ��������ã��ѶȲ���

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
6�������й��Ȼ�ѧ����ʽ��������ȷ���ǣ�������
| A�� | ��C��s��+O2��g��=CO2��g����H1��CO2��g��+C��s��=2CO��g����H2��2CO��g��+O2��g��=2CO2��g����H3����Ӧ�ȵĹ�ϵ����H1=��H2+��H3 | |
| B�� | ��2H2��g��+O2��g��=2H2O��1����H4��H2��g��+$\frac{1}{2}$O2��g��=H2O��1����H5����Ӧ�ȵĹ�ϵ����H4=��H5 | |
| C�� | ��2SO2��g��+O2��g��=2SO3��g����H��O�������ʵ��ȶ��ԣ�SO2��SO3 | |
| D�� | ������1molH2��g���е�H-H��������1 molO2��g���еĹ��ۼ��ֱ���Ҫ����436 kJ��498 kJ������������H2O��g���е�1 mol H-O���ܷų�463kJ����������2H2��g��+O2��g��=2H2O��g����H=-482kJ��mol-1 |
11����ѧ�㷺Ӧ�����������������˵����ȷ���ǣ�������
| A�� | ���͡����ͺ�ֲ���Ͷ���̼�⻯���� | |
| B�� | �����Ǻ�������Һ����������Ӧ�������ƾ��� | |
| C�� | ���������м���ϡ�����ܷ���������Ӧ | |
| D�� | �������м��뵨������ʹ�����ʷ������� |
1�������й�����Ӧ�õ�˵��������ǣ�������
| A�� | ��ʳ����ϴ��ˮƿ�е�ˮ�� | |
| B�� | �����г��õľ������װ���Ǹ��ݽ������������ʶ���Ƶ� | |
| C�� | ���������������������� | |
| D�� | Ϊ���ӳ���ʵ��ij����ڣ����ý��ݹ����������Һ�Ĺ�������ˮ�����������ϩ |
8������ָ����Ӧ�����ӷ���ʽ��ȷ���ǣ�������
| A�� | ������ͭ��Һ�м���NaHS��Һ��Cu2++HS-=CuS��+H+ | |
| B�� | �ô����ȥˮ���е�̼��ƣ�CaCO3+2H+=Ca2++H2O+CO2�� | |
| C�� | ��ǿ����Һ���չ�ҵ��ȡ����β����NO+NO2+2OH-=2NO3-+H2O | |
| D�� | ������SO2����ͨ�백ˮ�У�SO2+NH3•H2O=NH4++HSO3- |
5�����н����У������������������Һ���ܷų��������ǣ�������
| A�� | Al | B�� | Cu | C�� | Fe | D�� | Mg |
6����Ȼ��֬�ṹ�ĺ���Ϊ���ͣ���һ�����õķ���Ȼ��֬����ṹ�ĺ�����Ϊ���ǣ�C12H22O11�����÷���Ȼ��֬����ֱ���͵IJ��������ᣨC17H33COOH�������Ƿ�Ӧ���ã��䷴Ӧʾ��ͼ��ͼ��ʾ��ע��ͼ�еķ�Ӧʽ����������
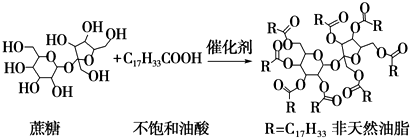
����˵����ȷ���ǣ�������

����˵����ȷ���ǣ�������
| A�� | ������Ҳ�Ǹ�֬����ĸ�������������֬������ | |
| B�� | �÷���Ȼ��֬������������Һ���ȣ���ˮ����ﲻ����ˮ��Ӧ | |
| C�� | ����Ȼ��֬Ϊ�߷��ӻ����� | |
| D�� | ����������ϡ�����������ˮ�⣬���տ����������л������� |
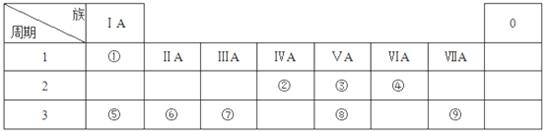
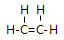 ���ɢܺ͢�����Ԫ����ɵĻ�����ĵ���ʽΪ
���ɢܺ͢�����Ԫ����ɵĻ�����ĵ���ʽΪ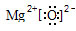 ��Ԫ�آ��ԭ�ӽṹʾ��ͼΪ
��Ԫ�آ��ԭ�ӽṹʾ��ͼΪ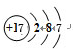 ��
��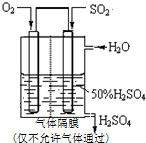 ��֪��SO2��g��+$\frac{1}{2}$O2��g��?SO3��g����H=-98kJ•mol-1��
��֪��SO2��g��+$\frac{1}{2}$O2��g��?SO3��g����H=-98kJ•mol-1��