��Ŀ����
 ��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺��1����Ũ������HCl�����ʵ���Ũ��Ϊ
11.9
11.9
mol?L-1����2��ȡ����������ĸ�������Һʱ�������������в�����ȡ����Ķ��ٶ��仯����
BD
BD
��A����Һ��HCl�����ʵ��� B����Һ��Ũ��
C����Һ��Cl-����Ŀ D����Һ���ܶ�
��3��ijѧ����������Ũ���������ˮ����500mL���ʵ���Ũ��Ϊ0.400mol?L-1��ϡ���ᣮ
�ٸ�ѧ����Ҫ��ȡ
16.8
16.8
mL����Ũ����������ƣ��������ƹ����У�����ʵ������������Ƶ�ϡ��������ʵ���Ũ���к�Ӱ�죿������������A��ʾ��ƫ����B��ʾ��ƫС������C��ʾ����Ӱ�족����
������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ��
A
A
�����ݺ���ҡ�ȡ����ã�����Һ���½����ټ�����������ˮ
B
B
����������Һ���õ�����ƿϴ����û�к��
C
C
���۸�36.5%��Ũ���ᣬ�������������ˮϡ�ͺ���������
����
����
18.25%��������������ˮϡ�ͺ�������������
����
18.25%���á�С�ڡ����ڡ����ڡ�����գ���������1���������ʵ���Ũ�ȵļ��㹫ʽc=
���м��㣻
��2�����ݸ����ļ��㹫ʽ�Ƿ�������й��жϣ�
��3���ٸ�����Һϡ�ͼ��㹫ʽ��c1V1=c2V2���㣻
�ڸ���ʵ�������c=
�IJ�����Ӱ����жϣ�
�ۼ����������ˮϡ�ͺ����ʵ��������䣬��Һ����������������2��������������ˮϡ�ͺ����ʵ��������䣬���ʵ�����С��ԭ����Һ������2����
| n |
| V |
��2�����ݸ����ļ��㹫ʽ�Ƿ�������й��жϣ�
��3���ٸ�����Һϡ�ͼ��㹫ʽ��c1V1=c2V2���㣻
�ڸ���ʵ�������c=
| n |
| V |
�ۼ����������ˮϡ�ͺ����ʵ��������䣬��Һ����������������2��������������ˮϡ�ͺ����ʵ��������䣬���ʵ�����С��ԭ����Һ������2����
����⣺��1������ͼʾ�����ݣ��������Ũ��Ϊ��c=
=
mol/L=11.9mol/L��
�ʴ�Ϊ��11.9��
��2��A��n=c?V��������Һ��HCl�����ʵ�������Һ����йأ���A����
B����Һ��Ũ���Ǿ�һ�ȶ��ģ�����ȡ��Һ������أ���B��ȷ��
C��N=n?NA=c?V?NA����������Һ����йأ���C����
D����Һ���ܶ��Ǿ�һ�ģ���������ȡ��Һ������أ���D��ȷ��
��ѡ��BD��
��3����c1V1=c2V2��
11.9mol/L��V1=0.400mol?L-1��0.5L��
����V1=0.0168L=16.8mL
�ʴ�Ϊ��16.8��
������������A��ʾ��ƫ����B��ʾ��ƫС������C��ʾ����Ӱ�족��
�����ӹ۲찼Һ�棬��ȡ��Һ�����ƫ������Ũ��ƫ�ߣ�
��ѡ��A��
���ټ�������ˮ���������Ƶ���Һ�����ƫ����ҺŨ��ƫС��
��ѡ��B��
��������Һ���õ�����ƿϴ����û�к�ɣ����ڶ���ʱ����Ҫ��������ˮ����������ƿ��������ˮ��Ӱ�����ƽ����
��ѡ��C��
�۸�36.5%��Ũ���ᣬ�������������ˮϡ�ͺ��������ʵ��������䣬��Һ��������Ϊ����������2�����������ʵ�������������������һ�룬��36.5%��
=18.25%��
������������ˮϡ�ͺ����ʵ��������䣬ˮ���ܶ�С��������ܶȣ����Ե������ˮ������С�ڵ�������������������Ϻ����ʵ��������䣬��Һ������С������������һ�룬����������������18.25%��
�ʴ�Ϊ�����ڣ� ���ڣ�
| n |
| V |
| 1000��1.19��36.5% |
| 36.5 |
�ʴ�Ϊ��11.9��
��2��A��n=c?V��������Һ��HCl�����ʵ�������Һ����йأ���A����
B����Һ��Ũ���Ǿ�һ�ȶ��ģ�����ȡ��Һ������أ���B��ȷ��
C��N=n?NA=c?V?NA����������Һ����йأ���C����
D����Һ���ܶ��Ǿ�һ�ģ���������ȡ��Һ������أ���D��ȷ��
��ѡ��BD��
��3����c1V1=c2V2��
11.9mol/L��V1=0.400mol?L-1��0.5L��
����V1=0.0168L=16.8mL
�ʴ�Ϊ��16.8��
������������A��ʾ��ƫ����B��ʾ��ƫС������C��ʾ����Ӱ�족��
�����ӹ۲찼Һ�棬��ȡ��Һ�����ƫ������Ũ��ƫ�ߣ�
��ѡ��A��
���ټ�������ˮ���������Ƶ���Һ�����ƫ����ҺŨ��ƫС��
��ѡ��B��
��������Һ���õ�����ƿϴ����û�к�ɣ����ڶ���ʱ����Ҫ��������ˮ����������ƿ��������ˮ��Ӱ�����ƽ����
��ѡ��C��
�۸�36.5%��Ũ���ᣬ�������������ˮϡ�ͺ��������ʵ��������䣬��Һ��������Ϊ����������2�����������ʵ�������������������һ�룬��36.5%��
| 1 |
| 2 |
������������ˮϡ�ͺ����ʵ��������䣬ˮ���ܶ�С��������ܶȣ����Ե������ˮ������С�ڵ�������������������Ϻ����ʵ��������䣬��Һ������С������������һ�룬����������������18.25%��
�ʴ�Ϊ�����ڣ� ���ڣ�
���������⿼�������ʵ���Ũ�ȵ��йؼ��㼰����һ�����ʵ���Ũ�ȵ���Һ���������ѶȲ���Ҫע������һ�����ʵ���Ũ����Һ���������������������c=
������Ӱ���жϣ������仯�����������Ӷ�ȷ��Ũ�ȵı仯��
| n |
| V |

��ϰ��ϵ�д�
�����Ŀ
 ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺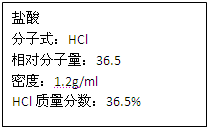 ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
 ��ͼΪʵ����ijŨ�����Լ�ƿ�ı�ǩ�ϵ��й����ݣ��Իش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ı�ǩ�ϵ��й����ݣ��Իش��������⣺ ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺