��Ŀ����
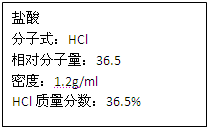 ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺��1����Ũ������HCl�����ʵ���Ũ��Ϊ
12mol/L
12mol/L
mol/L��ijѧ����������Ũ���������ˮ����500mL���ʵ���Ũ��Ϊ0.3mol/Lϡ���
��2����ѧ����Ҫ��ȡ
12.5
12.5
mL����Ũ����������ƣ���3�����ƹ����У�����Ҫʹ���ձ�����Ͳ���������⣬����Ҫʹ�õ������ǣ���д���ƣ�
��ͷ�ι�
��ͷ�ι�
��500mL����ƿ
500mL����ƿ
����4������ʱ������ȷ�IJ���˳���ǣ�Ҫ������ĸ��ʾ��ÿ����ĸֻ����һ�Σ�
BCAFED
BCAFED
��A����30mL����ˮϴ���ձ�2-3�Σ�ϴ��Һ��ע������ƿ����
B������Ͳȷ��ȡ����Ũ���������������ر���ע��ʢ����������ˮ��Լ30mL�����ձ��У��ò���������������ʹ���Ͼ���
C��������ȴ�������ز�����ע��500mL������ƿ��
D��������ƿ�ǽ����ߵ�ҡ��
E�����ý�ͷ�ιܼ�����ˮ��ʹ��Һ����ǡ����̶�������
F������������ƿ��С�ļ�����ˮ��ֱ��Һ��ӽ�����1-2cm��
��5�������ƹ����У�����ʵ�������ʹ�����Ƶ�ϡ��������ʵ���Ũ��ƫ�ߵ���
BD
BD
A��ҡ�Ⱥ��ã�����Һ����ڿ��ߣ�������ˮ�����������
B����Һע������ƿǰû�лָ������¾ͽ��ж���
C������ʱ���ӿ���
D��������ǰ����֪Ũ�ȵ�ϡ������ϴ����ƿ��
��������1�������1L��Һ���Ȼ�������ʵ������͵����Ȼ����Ũ�ȣ�
��2����������500mL���ʵ���Ũ��Ϊ0.3mol/Lϡ������Ҫ���Ȼ�������ʵ��������Ũ����������
��3����������һ�����ʵ���Ũ�ȵ���Һ�IJ���ѡ��������
��4������������Һʱ����������Ϊ����ȡ���ܽ⡢��Һ��ϴ�ӡ����ݡ��Ȳ�����������
��5������c=
=
�����жϣ����mƫС��Vƫ����cƫС�����mƫ���VƫС����cƫ�ݴ˷�����
��2����������500mL���ʵ���Ũ��Ϊ0.3mol/Lϡ������Ҫ���Ȼ�������ʵ��������Ũ����������
��3����������һ�����ʵ���Ũ�ȵ���Һ�IJ���ѡ��������
��4������������Һʱ����������Ϊ����ȡ���ܽ⡢��Һ��ϴ�ӡ����ݡ��Ȳ�����������
��5������c=
| n |
| V |
| m |
| MV |
����⣺��1��1L��Һ�к����Ȼ�������ʵ���ΪΪ��
=12mol����Ũ������HCl�����ʵ���Ũ��Ϊ12mol/L��
�ʴ�Ϊ��12mol/L��
��2��������Һϡ��ǰ�����ʵ����ʵ��������c1V1=c2V2��V1=
=0.0125L=12.5mL��
�ʴ�Ϊ��12.5mL��
��3������һ�����ʵ�����Ũ�ȵ���Һ��Ҫ�������У��ձ�����Ͳ�������������ж�����Ҫ��ͷ�ιܡ�����500mL��Һ��Ҫѡ��500mL����ƿ��
�ʴ�Ϊ����ͷ�ι� 500ml����ƿ��
��4��������Һʱ����������Ϊ����ȡ���ܽ⡢��Һ��ϴ�ӡ����ݡ��Ȳ�������ȷ��˳��Ϊ��BCAFED��
�ʴ�Ϊ��BCAFED��
��5��A��ҡ�Ⱥ��ã�����Һ����ڿ��ߣ�������ˮ����������У��������Ƶ���Һ���ƫ��Ũ��ƫС����A����
B����Һע������ƿǰû�лָ������¾ͽ��ж��ݣ���ȴ����Һ�����ƫС�����Ƶ���Һ��Ũ��ƫ�ߣ���B��ȷ��
C������ʱ���ӿ��ߣ��������Ƶ���Һ���ƫ��Ũ��ƫС����C����
D��������ǰ����֪Ũ�ȵ�ϡ������ϴ����ƿ���������Ƶ���Һ�����ʵ����ʵ���ƫ�������Ƶ���ҺŨ��ƫ�ߣ���D��ȷ��
��ѡBD��
| 1000mL��1.2g/mL��36.5% |
| 36.5g/mol |
�ʴ�Ϊ��12mol/L��
��2��������Һϡ��ǰ�����ʵ����ʵ��������c1V1=c2V2��V1=
| 0.5L��0.3mol/L |
| 12mol/L |
�ʴ�Ϊ��12.5mL��
��3������һ�����ʵ�����Ũ�ȵ���Һ��Ҫ�������У��ձ�����Ͳ�������������ж�����Ҫ��ͷ�ιܡ�����500mL��Һ��Ҫѡ��500mL����ƿ��
�ʴ�Ϊ����ͷ�ι� 500ml����ƿ��
��4��������Һʱ����������Ϊ����ȡ���ܽ⡢��Һ��ϴ�ӡ����ݡ��Ȳ�������ȷ��˳��Ϊ��BCAFED��
�ʴ�Ϊ��BCAFED��
��5��A��ҡ�Ⱥ��ã�����Һ����ڿ��ߣ�������ˮ����������У��������Ƶ���Һ���ƫ��Ũ��ƫС����A����
B����Һע������ƿǰû�лָ������¾ͽ��ж��ݣ���ȴ����Һ�����ƫС�����Ƶ���Һ��Ũ��ƫ�ߣ���B��ȷ��
C������ʱ���ӿ��ߣ��������Ƶ���Һ���ƫ��Ũ��ƫС����C����
D��������ǰ����֪Ũ�ȵ�ϡ������ϴ����ƿ���������Ƶ���Һ�����ʵ����ʵ���ƫ�������Ƶ���ҺŨ��ƫ�ߣ���D��ȷ��
��ѡBD��
���������⿼�������ʵ���Ũ�ȵ��йؼ��㼰����һ�����ʵ���Ũ�ȵ���Һ��֪ʶ�㣬�ѶȲ���Ҫע������һ�����ʵ���Ũ����Һ��������������C=
�жϣ������仯�����������Ӷ�ȷ��Ũ�ȵı仯��
| n |
| V |

��ϰ��ϵ�д�
�����Ŀ
 ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
 ��ͼΪʵ����ijŨ�����Լ�ƿ�ı�ǩ�ϵ��й����ݣ��Իش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ı�ǩ�ϵ��й����ݣ��Իش��������⣺ ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺