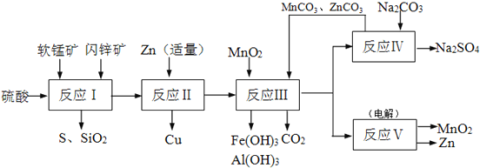
��Ŀ����
����Ŀ�������ڱ���1~36��֮���W��X��Y��Z��Q����Ԫ�أ����ǵ�ԭ�������������� ��֪W����������Ԫ�ؼȲ�ͬ����Ҳ��ͬ���壻X��Z�Ļ�̬ԭ�ӵĺ�����Ӿ��Ų���3���ܼ��ϣ��Ҿ���2��δ�ɶԵ��ӣ�QԪ��ԭ��������Ϊ29��
(1)�������Ų���QԪ�������ڱ�����____________����
(2)��һ������Y______Z���縺��Y______Z(������������������������)��
(3)W��Z�γɵij�����������W2Z��W2Z2��W��Y���γɶ��ֶ�Ԫ�������YW3��Y2W4��Y3W5��Y4W6������W2Z������Zԭ�ӵ��ӻ�����Ϊ______��YW3���ӵ����幹��Ϊ_____��YW3��������W2Z����Ҫԭ����___________________��(д�����㼴��)
(4)Q+��̬��������Ų�ʽΪ________________________________��
(5)Q+��Y3-�γɵľ����ṹ��ͼ��ʾ�����������Ӽ�ĺ˼��Ϊ a pm�������ӵ�������NA ��ʾ��������ܶ�Ϊ_____________g��cm-3��
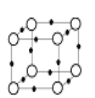
o-Y3- -Q+
���𰸡�ds �� �� sp3 ������ ���Ǽ��Է��ӣ��������ܣ�NH3��H2O���Ӽ���γ������NH3��H2O������Ӧ [Ar]3d10��1s22s22p63s23p63d10 (3��64+14)/[NA��(2a��10-10) 3 ]
��������
X��Z�Ļ�̬ԭ�ӵĺ�����Ӿ��Ų���3���ܼ��ϣ��Ҿ���2��δ�ɶԵ��ӣ�����X�ĵ����Ų�ʽΪ1s22s22p2��XΪC��Y�����Ų�ʽΪ1s22s22p4��ZΪO������X��Y��Zԭ������������������YΪN��QԪ��ԭ��������Ϊ29��QΪCu��W����������Ԫ�ؼȲ�ͬ����Ҳ��ͬ���壬��W��X��Y��Z��Q����Ԫ�أ����ǵ�ԭ��������������WΪH��
��1�������Ϸ���֪��QΪCu���������Ų���CuԪ�������ڱ�����ds����
�������ds��
��2����ԭ�Ӻ������2p�ܼ����ڰ���״̬�����Ե�һ������Y![]() Z��ͬ���ڴ����ҵ縺���������Ե縺��Y
Z��ͬ���ڴ����ҵ縺���������Ե縺��Y![]() Z��
Z��
�������![]() ��
��![]() ��
��
��3���ɷ�����֪��W2Z���Ӽ�H2O��H2O��Oԭ�ӵ��ӻ�����Ϊsp3��YW3���Ӽ�NH3���ӣ�NH3�����幹��Ϊ�����Σ�YW3��������W2Z����Ҫԭ���У����Ǽ��Է��ӣ��������ܣ�NH3��H2O���Ӽ���γ������������ˮ��Ӧ����һˮ�ϰ���
�����Ϊ��sp3�������Σ����Ǽ��Է��ӣ��������ܣ�NH3��H2O���Ӽ���γ������NH3��H2O������Ӧ��
��4��QΪCu��Cu+��̬��������Ų�ʽΪ: 1s22s22p63s23p63d10��[Ar]3d10��
�������1s22s22p63s23p63d10��[Ar]3d10��
��5��Cu+��N3-�γɵľ����ṹ��ͼ�����������ӵĺ˼��Ϊapm�����߳�Ϊ2apm���þ�����N3-�� 8![]() =1��Cu+��12
=1��Cu+��12![]() =3������Ļ�ѧʽΪCu3N���þ�����ܶ�
=3������Ļ�ѧʽΪCu3N���þ�����ܶ�![]()
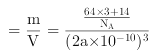 =(3��64+14)/[NA��(2a��10-10) 3 ] g��cm-3��
=(3��64+14)/[NA��(2a��10-10) 3 ] g��cm-3��
�������(3��64+14)/[NA��(2a��10-10) 3 ]��