��Ŀ����
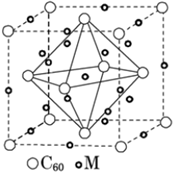
14��ʯī������ϩC60���ͽ��ʯ�Ľṹģ����ͼ��ʾ��ʯī����ʾ�����е�һ��ṹ����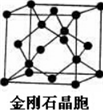
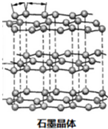
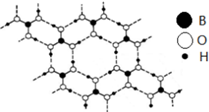
��1��ʯī��״�ṹ�У������֮��������Ϊ���»�����Ƭ����ƽ��ÿ����Ԫ����̼ԭ����Ϊ2�����ڲ�״�ṹ�У�ƽ��ÿ����������̼ԭ����2���������ᣨH3BO3����һ��Ƭ��״�ṹ��ɫ���壬����ʯī���ƵIJ�״�ṹ�����ڵ�H3BO3����ͨ�������������ͼ����������Bԭ���ӻ����������Ϊsp2��1mol H3BO3�ľ�������3mol���
��2���ɽ��ʯ������֪��ÿ�����ʯ����ռ��8��̼ԭ�ӣ�̼ԭ���ӻ�����Ϊsp3���ڸþ���1mol̼ƽ�����γ�2molC-C�����黯�����˹��ϳɵ����Ͱ뵼����ϣ��侧��ṹ����ʯ���ƣ�GaAs�����У�ÿ��As��4��Ga�������ҵ�һ������As��Ga�����������������=����
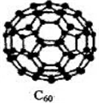
��3��C60���ڷ��� ���ԭ�ӡ����ӡ������壮����ṹ�У�C60������ÿ��̼ԭ��ֻ�����ڵ�3��̼ԭ���γɻ�ѧ����1mol C60�����ЦҼ�����ĿΪ90NA����ѧ�Ұ�C60��M������һ��������Ļ�������г������ܣ��侧����ͼ��ʾ��Mλ�ھ���������ڲ����û������е�Mԭ�Ӻ�C60���ӵĸ�����Ϊ3��1��
���� ��1��ʯī��״�ṹ�У������֮��������Ϊ���»�����Ƭ����ƽ��ÿ����Ԫ����̼ԭ����=$\frac{1}{3}$��6��
�ڲ�״�ṹ�У�ƽ��ÿ����������̼ԭ����=12��$\frac{1}{6}$��
����ͼ֪�������ᣨH3BO3����ÿ��Bԭ������3��Oԭ���Ҳ����µ��Ӷԣ��ݴ�ȷ��Bԭ���ӻ���ʽ��
1��������������γ�3��������ݴ˼���1mol H3BO3�ľ�����������ʵ�����
��2�����ʯ������Cԭ�Ӹ���=4+8��$\frac{1}{8}$+6��$\frac{1}{2}$��ÿ��Cԭ���γ��ĸ����ۼ����Ҳ����µ��Ӷԣ���Cԭ��Ϊsp3�ӻ������ʯ��ÿ��Cԭ���γ�̼̼������=4��$\frac{1}{2}$=2��GaAs����ṹ�ͽ��ʯ���ƣ�����GaAs�У�ÿ��As��4��Ga������ͬһ����Ԫ�أ�Ԫ�ص�һ����������ԭ��������������������ƣ�����IIA����VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ�
��3���ɷ��ӹ��ɵľ����Ƿ��Ӿ��壻C60������ÿ��̼ԭ��ֻ�����ڵ�3��̼ԭ���γɻ�ѧ����ÿ��Cԭ�Ӻ���1.5���Ҽ���
�þ�����Mԭ�Ӹ���=8+1+12��$\frac{1}{4}$=12��C60���Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4������M��C60����֮��=12��4=3��1��
��� �⣺��1��ʯī��״�ṹ�У������֮��������Ϊ���»�����Ƭ����ƽ��ÿ����Ԫ����̼ԭ����=$\frac{1}{3}$��6=2��
�ڲ�״�ṹ�У�ƽ��ÿ����������̼ԭ����=12��$\frac{1}{6}$=2��
����ͼ֪�������ᣨH3BO3����ÿ��Bԭ������3��Oԭ���Ҳ����µ��Ӷԣ��ݴ�ȷ��Bԭ���ӻ���ʽΪsp2��
1��������������γ�3���������1mol H3BO3�ľ�����������ʵ�����3mol��
�ʴ�Ϊ�����»�����2��2��sp2��3��
��2�����ʯ������Cԭ�Ӹ���=4+8��$\frac{1}{8}$+6��$\frac{1}{2}$=8��ÿ��Cԭ���γ��ĸ����ۼ����Ҳ����µ��Ӷԣ���Cԭ��Ϊsp3�ӻ������ʯ��ÿ��Cԭ���γ�̼̼������=4��$\frac{1}{2}$=2��GaAs����ṹ�ͽ��ʯ���ƣ�����GaAs�У�ÿ��As��4��Ga������ͬһ����Ԫ�أ�Ԫ�ص�һ����������ԭ��������������������ƣ�����IIA����VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ����Ե�һ������As��Ga��
�ʴ�Ϊ��8��sp3��2��4������
��3���ɷ��ӹ��ɵľ����Ƿ��Ӿ��壻C60������ÿ��̼ԭ��ֻ�����ڵ�3��̼ԭ���γɻ�ѧ����ÿ��Cԭ�Ӻ���1.5���Ҽ���1mol C60�����ЦҼ�����ĿΪ90NA��
�þ�����Mԭ�Ӹ���=8+1+12��$\frac{1}{4}$=12��C60���Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4������M��C60����֮��=12��4=3��1��
�ʴ�Ϊ�����ӣ�90NA��3��1��
���� ���⿼�����ʽṹ�����ʣ�Ϊ��Ƶ���㣬�漰�������㡢ԭ���ӻ���ʽ�жϡ������ܴ�С�жϵ�֪ʶ�㣬�ѵ��Ǿ������㣬ע�⣨3����ԭ�Ӹ������㣬ͬʱ����ռ�������������Ŀ�Ѷ��еȣ�

| ��Ӧǰ | ��Ӧ�� | ||
| ʵ�� ���� | �ձ���ϡ��������� | ʯ��ʯ��Ʒ������ | �ձ������л��������� |
| 134.4 g | 10 g | 141.1 g | |
��1����ʯ��ʯ��̼��Ƶ�����������
��2���μӷ�Ӧ10%�������������
| A�� | ��֪��������������ͨʽXOm��OH��n����ʾ��H2SeO3��HMnO4��H3BO3��H3PO4 ��������������ǿ����H2SeO3 | |
| B�� | HF�ķе��H2O������ˮ������ļ��ܱȷ�����������ܴ� | |
| C�� | ����������ˮ������ͭ��Һ�м����Ҵ�������������ɫ������������Ϊ�Ҵ����Ӽ��Ա�ˮС | |
| D�� | H2CO3�����еķ��ǻ����ĸ���Ϊ1��Ȼ�������Խ�����ԭ��������ˮ�Ķ�����̼����ֻ�м�������ˮ��ϳ�̼�� |
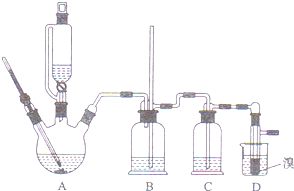 ��֪ʵ�����Ʊ�1��2-����������ܴ��ڵ���Ҫ����Ӧ�У��Ҵ���Ũ����Ĵ�������l40����ˮ�������ѣ�
��֪ʵ�����Ʊ�1��2-����������ܴ��ڵ���Ҫ����Ӧ�У��Ҵ���Ũ����Ĵ�������l40����ˮ�������ѣ�����������������Ҵ��Ʊ�1��2-���������װ������ͼ��ʾ���й������б����£�
| �Ҵ� | 1��2-�������� | ���� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g•cm-3 | 0.79 | 2.2 | 0.71 |
| �е�/�� | 78.5 | 132 | 34.6 |
| �۵�/�� | һl30 | 9 | -1l6 |
��1����Ӧԭ����CH3CH2OH$��_{170��}^{Ũ����}$CH2=CH2��+H2O��CH2=CH2+Br2��BrCH2CH2Br
��2����װ��C��Ӧ����c��������ȷѡ��ǰ����ĸ����Ŀ�������շ�Ӧ�п������ɵ���������
a��ˮ b��Ũ���� c������������Һ d������̼��������Һ
��3���жϸ��Ƹ���Ӧ�Ѿ�������������������ɫ��ȫ��ȥ��
��4�������������������������ѣ���������ķ�����ȥ��
��5����Ӧ������Ӧ����ˮ��ȴװ��D������ҪĿ������ȴ�ɱ�����Ĵ����ӷ������ֲ��ܹ�����ȴ�����ñ�ˮ������ԭ���Dz�Ʒ1��2-����������۵㣨���̵㣩�ͣ�������ȴ�����̶��������ܣ�
 ���������仯�����ںϽ�����Լ������ȷ���Ӧ�ù㷺��
���������仯�����ںϽ�����Լ������ȷ���Ӧ�ù㷺�� ����HCHO������̼ԭ�Ӳ�ȡsp2�ӻ��ķ����Т٢ۢܣ���������ţ���HCHO���ӵ�����ṹΪƽ�������Σ�
����HCHO������̼ԭ�Ӳ�ȡsp2�ӻ��ķ����Т٢ۢܣ���������ţ���HCHO���ӵ�����ṹΪƽ�������Σ�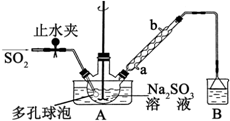 ���������Ƽ�ȩ��NaHSO2•HCHO•2H2O���׳Ƶ��飬���ȶ���120��ʱ��ֽ⣮��ӡȾ��ҽҩ�Լ�ԭ���ܹ�ҵ���й㷺Ӧ�ã���Na2SO3��SO2��HCHO��п��Ϊԭ���Ʊ����������Ƽ�ȩ��ʵ�鲽����ͼ��
���������Ƽ�ȩ��NaHSO2•HCHO•2H2O���׳Ƶ��飬���ȶ���120��ʱ��ֽ⣮��ӡȾ��ҽҩ�Լ�ԭ���ܹ�ҵ���й㷺Ӧ�ã���Na2SO3��SO2��HCHO��п��Ϊԭ���Ʊ����������Ƽ�ȩ��ʵ�鲽����ͼ��