��Ŀ����
�����г������ؽ�����Ⱦ���У�����Ǧ���̡������ӡ�������ҵ��ˮ�к��е�Cr2O72-��CrO42-�����õķ��������֡�
����1����ԭ������
�÷��Ĺ�������Ϊ ��
��
���еڢٲ�����ƽ��2CrO42-����ɫ����2H�� Cr2O72-����ɫ����H2O��
Cr2O72-����ɫ����H2O��
��1��д���ڢٲ���Ӧ��ƽ�ⳣ������ʽ_________________________________��
��2�����ڵڢٲ���Ӧ������˵����ȷ����________��
A��ͨ���ⶨ��Һ��pH�����жϷ�Ӧ�Ƿ��Ѵ�ƽ��״̬
B���÷�ӦΪ������ԭ��Ӧ
C��ǿ���Ի�������Һ����ɫΪ��ɫ
��3���ڢڲ��У���ԭ0.1 mol Cr2O72-����Ҫ________mol��FeSO4��7H2O��
��4���ڢ۲�������Cr��OH��3�⣬���������ɵij���Ϊ________������Һ�д������³����ܽ�ƽ�⣺Cr��OH��3��s�� Cr3����aq����3OH����aq���������£�Cr��OH��3���ܶȻ�Ksp��10��32����c��Cr3��������10��5 mol��L��1ʱ����Ϊc��Cr3�����Ѿ���ȫ�������ֽ��ڢ۲���Һ��pH����4����ͨ������˵��Cr3���Ƿ������ȫ����д��������̣���____________________________________________________________________________��
Cr3����aq����3OH����aq���������£�Cr��OH��3���ܶȻ�Ksp��10��32����c��Cr3��������10��5 mol��L��1ʱ����Ϊc��Cr3�����Ѿ���ȫ�������ֽ��ڢ۲���Һ��pH����4����ͨ������˵��Cr3���Ƿ������ȫ����д��������̣���____________________________________________________________________________��
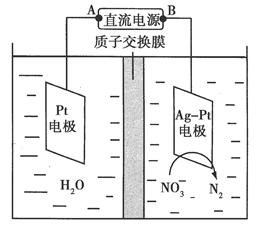
����2����ⷨ
��5��ʵ����������ͼװ��ģ���ⷨ������Cr2O72-�ķ�ˮ�����ʱ������ӦʽΪ________��������ӦʽΪ________���õ��Ľ������������������ɳ�����ȫ����ˮ�ĵ���ƽ��ǶȽ�����ԭ����___________________________________________________________��
��1��K��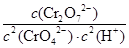
��2��AC
��3��0.6
��4��Fe��OH��3����pH����4ʱ��c��OH������10��10 mol��L��1��c��Cr3������10��32/c3��OH������10��2 mol��L��1��10��5 mol��L��1�����Cr3��û�г�����ȫ
��5��Fe��2e��=Fe2����2H����2e��=H2�����������ɵĽ����������������ƶ���������Ӧ������H����������ˮ�ĵ���ƽ�⣬�ٽ���ˮ�ĵ��룬ʹ��Һ��OH����Ũ��������Һ�ʼ���
����

����I2O5������CO��Ⱦ�����ⶨCO����ӦΪ��5CO��g����I2O5��s�� 5CO2��g����I2��s������H 1
5CO2��g����I2��s������H 1
��1����֪��2CO��g����O2��g�� 2CO2��g������H 2
2CO2��g������H 2
2I2��s����5O2��g�� 2I2O5��s������H 3
2I2O5��s������H 3
��H 1�� ���ú���H 2�ͦ�H 3�Ĵ���ʽ��ʾ����
��2����ͬ�¶��£���װ������I2O5�����2 L�����ܱ�������ͨ��2molCO�����CO2����������գ�CO2����ʱ��t�仯������ͼ����ش�
�ٴӷ�Ӧ��ʼ��a��ʱ�ķ�Ӧ����Ϊv(CO)�� ��b��ʱ��ѧƽ�ⳣ��Kb�� ��
��d��ʱ���¶Ȳ��䣬�����������ѹ����ԭ����һ�룬����ͼ�в��仭��CO2��������ı仯���ߡ�
������˵����ȷ���� ��������ĸ��ţ�
| A�������������ܶȲ��䣬������Ӧ�ﵽƽ��״̬ |
| B�������¶��£�c��ʱ��ϵ�л�������ƽ����Է���������� |
| C������I2O5��Ͷ�������������CO��ת���� |
| D��b���d��Ļ�ѧƽ�ⳣ����Kb��Kd |
��.��һ���Ϊ10 L�������У�ͨ��һ������CO��H2O����850��ʱ�������·�Ӧ��CO(g)��H2O(g) CO2(g)��H2(g)����H<0��CO��H2OŨ�ȱ仯��ͼ��ʾ��
CO2(g)��H2(g)����H<0��CO��H2OŨ�ȱ仯��ͼ��ʾ��
(1)0��4 min��ƽ����Ӧ����v(CO)��________mol/(L��min)����Ӧ�ڵ�5 minʱ��ƽ�ⳣ��K��________��
t��ʱ����Ũ��(mol/L)�ı仯
| ʱ��(min) | CO | H2O | CO2 | H2 |
| 0 | 0. 200 | 0. 300 | 0 | 0 |
| 2 | 0. 138 | 0.238 | 0.062 | 0.062 |
| 3 | c1 | c2 | c3 | c3 |
| 4 | c1 | c2 | c3 | c3 |
| 5 | 0. 116 | 0. 216 | 0. 084 | |
| 6 | 0. 096 | 0. 266 | 0. 104 | |
(2)t��(����850��)ʱ������ͬ�����з���������Ӧ�������ڸ����ʵ�Ũ�ȱ仯�����ʾ��
�ٱ���3��4 min֮�䷴Ӧ����________״̬��c1��ֵ________(�>����������<��)0.08 mol/L��
�ڷ�Ӧ��4��5 min�䣬ƽ�����淴Ӧ�����ƶ������ܵ�ԭ����________(���ţ���ͬ)������5��6 min֮����ֵ�����仯�����ܵ�ԭ����________��
A������ˮ����B�������¶�C��ʹ�ô���D����������Ũ��
��.���ȵ�ϡ�������ܽ���11.4 g�����������壬������50 mL 0.5 mol/L��KNO3��Һ��ʹ���е�Fe2��ȫ��ת����Fe3����KNO3Ҳ��ȫ��Ӧ���ų�NxOy���塣
(3)�����x��________��y��________��
(4)д���÷�Ӧ�Ļ�ѧ����ʽ��________________(x��y�þ�����ֵ��ʾ)��
(5)��Ӧ������������________��

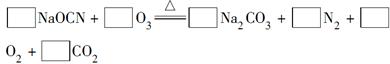

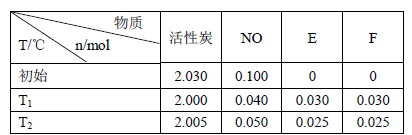
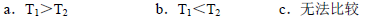
 ��
��  MnCl2+Cl2��+ 2H2O
MnCl2+Cl2��+ 2H2O