��Ŀ����
6����ͼ�е��������ʾ��ɶ�����Ԫ����ɣ����мס��ҡ�������Ϊ���ʣ������¼ס���Ϊ��ɫ���壬��Ϊ����ɫ���壮���dz����������㷺���ں��졢���չ�ҵ����ҵ�ϴӺ�ˮ����ȡG����ͨ�����G��ȡ����ͬʱ�õ������ﶡ��A��E�ķ����о���10�����ӣ�A��һ����ʹʪ��ĺ�ɫʯ����ֽ���������壬E�ڳ���������ɫ��ζ����Һ����ͼ�и�����ת�����漰����������ʡ�ԣ��ش��������⣺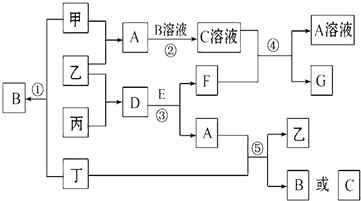
��1����Ӧ�١���������������ԭ��Ӧ���Ǣ٢ݣ�����ţ���
��2��д����Ӧ�۵Ļ�ѧ����ʽMg3N2+6H2O=3Mg��OH��2+2NH3����
��3��C��ˮ��Һ�����ԣ������ӷ���ʽ����NH4++H2O?NH3•H2O+H+��
��4������1mol��ʱת�Ƶ��ӵ���ĿΪ6NA ��
��5������C�������ӵķ�����ȡ������Һ���Թ��У��μ�����������Һ�����ȣ�����ʪ�ĺ�ɫʯ����ֽ�����Թܿ��д̼�����ζ������ų�����ʹ��ʪ�ĺ�ɫʯ����ֽ��������˵����笠����ӣ�
���� �����¼ס���Ϊ��ɫ���塢��Ϊ����ɫ���壬��ΪCl2�����dz����������㷺���ں��졢���չ�ҵ����ҵ�ϴӺ�ˮ����ȡGΪMgCl2����ͨ�����G��ȡ��ΪMg��ͬʱ�õ������ﶡ��Cl2����A��E�ķ��Ӿ���10�����ӣ�A��һ����ʹʪ��ĺ�ɫʯ����ֽ����������ΪNH3���ƶϼ��ҵ��ʻ������ɰ�����˵���ס����������͵�������������Ӧ�����Ȼ��⣬��֪����ΪH2����ΪN2��BΪHCl��DΪ Mg3N2��E����������ɫ��ζ��Һ��ΪH2O��CΪNH4Cl��FΪMg��OH��2��A+��=��+B��C�����ж�Ϊ��Cl2+NH3��N2+HCl��NH4Cl�����Դ˽����⣮
��� �⣺�����¼ס���Ϊ��ɫ���塢��Ϊ����ɫ���壬��ΪCl2�����dz����������㷺���ں��졢���չ�ҵ����ҵ�ϴӺ�ˮ����ȡGΪMgCl2����ͨ�����G��ȡ��ΪMg��ͬʱ�õ������ﶡ��Cl2����A��E�ķ��Ӿ���10�����ӣ�A��һ����ʹʪ��ĺ�ɫʯ����ֽ����������ΪNH3���ƶϼ��ҵ��ʻ������ɰ�����˵���ס����������͵�������������Ӧ�����Ȼ��⣬��֪����ΪH2����ΪN2��BΪHCl��DΪ Mg3N2��E����������ɫ��ζ��Һ��ΪH2O��CΪNH4Cl��FΪMg��OH��2��A+��=��+B��C�����ж�Ϊ��Cl2+NH3��N2+HCl��NH4Cl����
��1�������Ϸ�����֪��Ϊ�����������ķ�Ӧ��Ϊ������ԭ��Ӧ����Ϊ�������Ȼ���ķ�Ӧ������������ԭ��Ӧ����Ϊ����þ��ˮ�ⷴӦ������������ԭ��Ӧ����Ϊ�Ȼ����������þ�ķ�Ӧ��Ϊ���ֽⷴӦ���������Ͱ����ķ�Ӧ��Ϊ������ԭ��Ӧ��
�ʴ�Ϊ���٢ݣ�
��2��DΪ Mg3N2��EΪH2O��Mg3N2��H2O����ˮ�ⷴӦ����Ӧ�Ļ�ѧ����ʽΪMg3N2+6H2O=3Mg��OH��2+2NH3����
�ʴ�Ϊ��Mg3N2+6H2O=3Mg��OH��2+2NH3����
��3��CΪNH4Cl��笠�����ˮ��ʹ��Һ�����ԣ����ӷ���ʽΪNH4++H2O?NH3•H2O+H+��
�ʴ�Ϊ��NH4++H2O?NH3•H2O+H+��
��4����ΪCl2��AΪNH3����ΪN2�����ݵ��ӵ�ʧ�غ��֪������1molN2ʱ��Ԫ�ص���-3�۱�Ϊ0�ۣ�����ת�Ƶ��ӵ���ĿΪ6NA ��
�ʴ�Ϊ��6NA ��
��5��CΪNH4Cl������C�������Ӽ�笠����ӵķ�����ȡ������Һ���Թ��У��μ�����������Һ�����ȣ�����ʪ�ĺ�ɫʯ����ֽ�����Թܿ��д̼�����ζ������ų�����ʹ��ʪ�ĺ�ɫʯ����ֽ��������˵����笠����ӣ�
�ʴ�Ϊ��ȡ������Һ���Թ��У��μ�����������Һ�����ȣ�����ʪ�ĺ�ɫʯ����ֽ�����Թܿ��д̼�����ζ������ų�����ʹ��ʪ�ĺ�ɫʯ����ֽ��������˵����笠����ӣ�
���� ���⿼��������ת����ϵ���������ʵ��ۺ�Ӧ�ã�Ϊ��Ƶ���㣬��Ҫ����������������þ���������ʵ��ƶϺͷ�Ӧ����Ӧ�ã����ӷ���ʽ��д����ѧ����ʽ��д��֪ʶ����Ŀ�Ѷ��еȣ�

| A�� | 1 mol�Ҵ�ȼ������3 molˮ | |
| B�� | �Ҵ����������� | |
| C�� | 1 mol�Ҵ���������Na���õ�0.5 mol H2 | |
| D�� | �Ҵ�����������ȼ�ϣ���ȾС�������� |
| A�� | 1 | B�� | 2 | C�� | 3 | D�� | 4 |
| A�� | AlCl3��Һ�м��������İ�ˮ��Al3++3OH-�TAl��OH��3�� | |
| B�� | ����������Һ��ϡ H2SO4��Ba2++OH-+SO42-+H+�TBaSO4��+H2O | |
| C�� | ���Ȼ�������Һ��ͨ��������2Fe2++Cl2�T2Fe3++2Cl- | |
| D�� | �ƺ���ˮ��Ӧ��Na+2H2O=Na++2OH-+H2�� |
 ������ͼװ�ý���ʵ�飬��ʼʱ��a��b����Һ����ƽ���ܷ�ã�����һ��ʱ�䣮����˵������ȷ���ǣ�������
������ͼװ�ý���ʵ�飬��ʼʱ��a��b����Һ����ƽ���ܷ�ã�����һ��ʱ�䣮����˵������ȷ���ǣ�������| A�� | a����Һ��pH����b����Һ��pH��С | |
| B�� | һ��ʱ���a��Һ�����b��Һ�� | |
| C�� | a�ܷ���������ʴ��b�ܷ���������ʴ | |
| D�� | a��b����������ͬ�ĵ缫��Ӧʽ��Fe-2e-=Fe2+ |
 W��X��Y��Z���ֶ�����Ԫ����Ԫ�����ڱ��е����λ����ͼ��ʾ��������������֮�͵���24���ɴ˿�֪����˵��������ǣ�������
W��X��Y��Z���ֶ�����Ԫ����Ԫ�����ڱ��е����λ����ͼ��ʾ��������������֮�͵���24���ɴ˿�֪����˵��������ǣ�������| A�� | ԭ�Ӱ뾶��С��W��X | B�� | ��̬�⻯��ķе㣺Y��X | ||
| C�� | �������ӵĻ�ԭ�ԣ�Y��Z | D�� | ��̬�⻯����ȶ��ԣ�X��Y |
| A�� | ��������Һ�м�������İ�ˮ��Al3++4OH-=AlO2-+2H2O | |
| B�� | ̼����������Һ��Ӧ��CaCO3+2H+=Ca2++CO2��+H2O | |
| C�� | ������Ͷ��ˮ�У�2Na+2H2O=2Na++2OH-+H2�� | |
| D�� | �Ȼ�����Һ������Ӧ��Fe3++Fe=2 Fe2+ |
 ��
��