��Ŀ����
12��п��һ��Ӧ�ù㷺�Ľ�����Ŀǰ��ҵ����Ҫ���á�ʪ��������ұ��п��ij��п�����Ҫ�ɷ�ΪZnS����������FeS�������ɷ֣�������Ϊԭ��ұ��п�Ĺ����������£�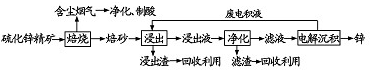
��ش��������⣮
��1����п����ı������������յķ���¯�н��У���Ҫ�ɷַ�����Ӧ�Ļ�ѧ����ʽΪ2ZnS+3O2$\frac{\underline{\;����\;}}{\;}$2ZnO+2SO2��
��2�����չ����в����ĺ��������ɾ������ᣬ��������ں����Ľ������������ղ�������ҵ���Ʊ������������Ҫ�ηֱ�Ϊ����¯�п�ʯȼ�����ɶ������Ӵ����д�������Ӧ�����������������������γ����ᣮ
��3������Һ�������������м������Ҫ����ΪZn�ۣ����������û���Fe�ȣ�
��4�������������е������������壬��������Pb-Ag�Ͻ���Ե缫��п�����������������ĵ缫��Ӧ����ʽΪ4OH--4e-=2H2O+O2����
��5���Ľ���пұ�����գ������ˡ���ѹ�������ȫʪ�����̣���ʡ�������¿�����Ⱦ�ı��չ��̣��ֿɻ��һ�ֹ�ҵ��ֵ�ķǽ������ʣ�����ѹ�����������Ҫ��Ӧ�����ӷ���ʽΪ2ZnS+4H++O2=2Zn2++2S��+2H2O��
��6���ҹ��Ŵ������á�������ұ��п��������Ӧ�����ġ��칤������й��ڡ�������Ǧ���ļ��أ���¯��ʯʮ�װ����һ����ڣ�����Ȼ�������ú̿����ʢ�������н�������Ѻ죬����������ٹ�ȡ������������ǦҲ��������п���չ�����Ҫ��Ӧ�Ļ�ѧ����ʽΪZnCO3+2C$\frac{\underline{\;����\;}}{\;}$Zn+3CO������ע��¯��ʯ����Ҫ�ɷ�Ϊ̼��п����Ǧ��ָ����п��
���� ��п����ı��տ�����ZnO���������ȣ������������к���������������Ʊ����ᣬ����Һ�����������������п�����������������п��ַ�Ӧ�����û���������Һ����Ҫ��������п�������ɵõ�п�����ᣬ���Һ�к������ᣬ��ѭ�����ã�
��1����п�����Ҫ�ɷ���ZnS�����������������������ԭ��Ӧ���жϱ�ɰ����Ҫ�ɷ�Ϊ����п��
��2���������ɵĺ����������ת��Ϊ���ᣬ��ҵ���Ʊ�����Ϊ����¯����ȼ�����ɶ������Ӵ����д�������Ӧ��������������������98.3%��Ũ�����������������γɷ������
��3���ú�п���л�����FeS�����ʣ�����������ת��Ϊ�������ӣ��ɼ���п�۳�ȥ�������ӣ��Ӷ���ȥFe��
��4�������������У��ǵ��ZnSO4��������������������ʧ���ӷ���������Ӧ��������п���ӵõ���������п���Դ���д�缫��Ӧ��
��5������ѹ�����������˼�壬��֪��Ӧ���к���H+��O2�����Ի�÷ǽ�������S�����ݷ�Ӧ���������д����ѧ����ʽ��
��6��������Ŀ������֪��Ӧ��ΪZnCO3��C�����ﺬ��Zn�����ݷ�Ӧ���������д����ѧ����ʽ��
��� �⣺��1����п�����Ҫ�ɷ���ZnS�����������������������ԭ��Ӧ����ɰ����Ҫ�ɷ�ΪZnO����Ӧ�Ļ�ѧ����ʽΪ��2ZnS+3O2$\frac{\underline{\;����\;}}{\;}$2ZnO+2SO2��
�ʴ�Ϊ��2ZnS+3O2$\frac{\underline{\;����\;}}{\;}$2ZnO+2SO2��
��2���������ɵĺ����������ת��Ϊ���ᣬ���ں����Ľ����������������������գ���ҵ���Ʊ�����Ϊ����¯����ȼ�����ɶ������Ӵ����д�������Ӧ��������������������98.3%��Ũ�����������������γɷ������
�ʴ�Ϊ���������������գ�����¯�п�ʯȼ�����ɶ������Ӵ��Ҵ����������������������������γ����
��3���ú�п���л�����FeS�����ʣ�����������ת��Ϊ�������ӣ��ɼ���п�۳�ȥ�������ӣ��Ӷ���ȥFe���ʴ�Ϊ��Zn�ۣ��û���Fe�ȣ�
��4�������������У��ǵ��ZnSO4������п���ӷŵ�����п����п��������������������������Ӧ������ΪO2���缫��ӦʽΪ��4OH--4e-=2H2O+O2����
�ʴ�Ϊ������4OH--4e-=2H2O+O2����
��5������ѹ�����������˼�壬��֪��Ӧ���к���H+��O2�����Ի�÷ǽ�������S��д����ѧ����ʽΪ��2ZnS+4H++O2=2Zn2++2S��+2H2O��
�ʴ�Ϊ��2ZnS+4H++O2=2Zn2++2S��+2H2O��
��6��������Ŀ������֪��Ӧ��ΪZnCO3��C�����ﺬ��Zn����ѧ����ʽΪ��ZnCO3+2C$\frac{\underline{\;����\;}}{\;}$Zn+3CO�����ʴ�Ϊ��ZnCO3+2C$\frac{\underline{\;����\;}}{\;}$Zn+3CO����
���� �����Ѷ��еȣ���������������������û��ϼ۵�ԭ�����ָ��Ԫ�صĻ��ϼ۵ķ�������ѧ����ʽ����д���������������ʵĻ�ѧ����ʽ�ļ��������ȷ�����Ĺؼ���

| A�� | NiMH ��طŵ�����У������ĵ缫��ӦʽΪ��NiOOH+H2O+e-=Ni��OH��2+OH- | |
| B�� | ��������OH-���Ӵ�����������Ǩ�� | |
| C�� | �������������ĵ缫��Ӧʽ��H2O+M+e-=MH+OH-��H2O�е�H��M��ԭ | |
| D�� | �ŵ���OH-���Ӵ�������Ǩ�� |
| A�� | NaHSO4����ˮ��NaHSO4?Na++H++SO42- | |
| B�� | ��������룺HClO�TClO-+H+ | |
| C�� | HF����ˮ��HF+H2O?H3O++F- | |
| D�� | NH4Cl����ˮ��NH4++H2O?NH3•H2O+H+ |
| A�� | ��ϵͳ��������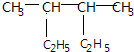 ��������2��3-���һ����� ��������2��3-���һ����� | |
| B�� | ������4��̼ԭ�ӣ�����̼ԭ������6����������10�� | |
| C�� | �ṹƬ��Ϊ �ĸ߾�������䵥��ͨ�����۷�Ӧ���ɵ� �ĸ߾�������䵥��ͨ�����۷�Ӧ���ɵ� | |
| D�� | ����������ϩ�;���ϩ��ȫȼ�գ������Ķ�����̼��������Ϊ1��1 |
| A�� | ��Ϣ��ҵ�еĹ��µ���Ҫ�ɷ��ǵ��ʹ� | |
| B�� | NO2��CO2��SO2��PM2.5�������ᵼ������ | |
| C�� | ��������ֲ��ϲ������Ͻ������Ͻ�ǿ�ȴ������ᡢ����ʴ����ǿ | |
| D�� | ���ͷ���ʹ���Ƴ��ĸ�����ɶ�ף�����Ϊ���ͷ��к���̼������ |
| A�� | FeCl2��NH4SCN��Һ�� | B�� | KI��������Һ�� | ||
| C�� | ���ף���ɫʯ����Һ�� | D�� | Na2SO3�� BaCl2 ��Һ�� |
| A�� | ����ʽΪC4H9Cl������һ����4�ֽṹ | |
| B�� | ����������KMnO4��Һ��ȥ�����е���ϩ | |
| C�� | 2��2-��������������̼ԭ����ͬһƽ���� | |
| D�� | CH3-CH=CH-CH3��HCl�����ӳɷ�Ӧ������������ |

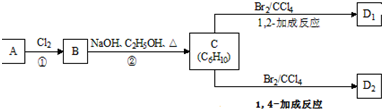
 ��
��