��Ŀ����
����Ŀ����
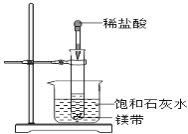
��1��ʵ�����ܹ۲쵽��������____________________����ѡ����ţ�
A���Թ���þƬ���ܽ� B���Թ��в�����ɫ����
C���ձ���ڱ��� D���ձ��ײ�����������ɫ����
��2����ʵ����֪��MgCl2��Һ��H2��������________������ڡ���С�ڡ������ڡ���þƬ���������������
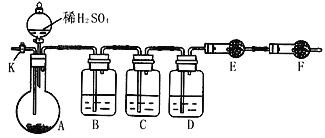
����50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺
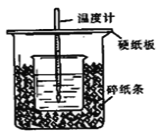
��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��___________������֮���һ���������____________________��
��2�����ձ����粻��Ӳֽ�壬��õ��к�����ֵ������ֵ��__________���ƫ��ƫС����Ӱ�족����
��1��úȼ�յķ�Ӧ�ȿ�ͨ����������;�������ã�a.����ú�ڳ���Ŀ�����ֱ��ȼ�ղ����ķ�Ӧ�ȣ�b.��ʹú��ˮ������Ӧ�õ�������һ����̼��Ȼ��ʹ�õ���������һ����̼�ڳ���Ŀ�����ȼ�ա����������̵��Ȼ�ѧ����ʽΪ��
a��C��s����O2��g��===CO2��g�� ��H��E1 ��
b��C��s����H2O��g��===CO��g����H2��g����H��E2 ��
H2��g����1/2O2��g��===H2O��g����H��E3 ��
CO��g����1/2O2��g��===CO2��g����H��E4 ��
�����E1��E2��E3��E4֮��Ĺ�ϵΪE2��_________________��
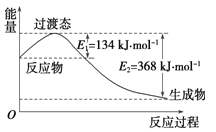
��2����ͼ��ʾ�ڳ��³�ѹ�£�1Ħ��NO2 ��1Ħ��CO��ȫ��Ӧ����CO2��NO�����������仯ʾ��ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ��___________________��
��3����ѧ��Ӧ���ʱ��뷴Ӧ���������ļ����йء���֪ijЩ��ѧ���ļ������±���ʾ��
���ۼ� | H��H | Cl��Cl | H��Cl |
����/��kJ��mol��1�� | 436 | 247 | 434 |
��Ӧ��H2��g��+Cl2��g��=2HCl��g�����ʱ䦤H �� ____________________��
���𰸡�A B DС�ڻ��β��������С�ձ��ںʹ��ձ���û��ƽ�루�������ʴ𰸸��֣�ƫСE2��E1��E3��E4NO2��g��+CO��g��=NO��g��+CO2��g�� ��H����234 kJ��mol��1��H ����185 kJ��mol��1
��������
��)(1)þ��������ҷ�Ӧ�������������ų��������ȣ������������Ƶ��ܽ�����¶����߶���С�����Ա���ʯ��ˮ���º���������������ʹ��Һ�ʻ���״��þ���ܽ⣬��ABDѡ���е�������ϣ��ʴ�Ϊ��ABD��
(2)����Ӧ����������������������ʱ����Ӧ�Ƿ��ȷ�Ӧ����MgCl2��Һ��H2��������С��þƬ����������������ʴ�Ϊ��С�ڣ�
��)(1)�����ȼƵĹ����֪��װ�õ�ȱ�������ǻ��β�����������к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹����������ձ�Ϊһ���ߣ���������ɢʧ�ʴ�Ϊ�����β����������С�ձ��ںʹ��ձ���û��ƽ�룻
(2)���ձ����粻��Ӳֽ�壬����һ��������ɢʧ����õ��к�����ֵ�����С���ʴ�Ϊ��ƫС��
��)(1)��C(s)+O2(g)�TCO2(g)��H=E1����H2(g)+![]() O2(g)�TH2O(g)��H=E3����CO(g)+
O2(g)�TH2O(g)��H=E3����CO(g)+![]() O2(g)�TCO2(g)��H=E4�����ݸ�˹���ɣ���-��-�ܿɵã�C(s)+H2O(g)�TCO(g)+H2(g)����E2=E1-E3-E4���ʴ�Ϊ��E1-E3-E4��
O2(g)�TCO2(g)��H=E4�����ݸ�˹���ɣ���-��-�ܿɵã�C(s)+H2O(g)�TCO(g)+H2(g)����E2=E1-E3-E4���ʴ�Ϊ��E1-E3-E4��
(2)��ͼ��֪��1Ħ��NO2��1Ħ��CO��ȫ��Ӧ����CO2��NO�ų�����Ϊ(368-134)kJ=234kJ����Ӧ�Ȼ�ѧ����ʽΪ��NO2(g)+CO(g)�TNO(g)+CO2(g)��H=-234 kJmol-1���ʴ�Ϊ��NO2(g)+CO(g)�TNO(g)+CO2(g)��H=-234 kJmol-1��
(3)��Ӧ��=��Ӧ���ܼ���-�������ܼ��ܣ��ʷ�Ӧ��H2(g)+Cl2(g)�T2HCl(g)���ʱ���H=436kJ/mol+247kJ/mol-2��434kJ/mol=-185kJ/mol���ʴ�Ϊ��-185 kJ/mol��