题目内容
(12分)A、B、C、D、E、F为六种20号以前的元素,其核电荷数依次增大,A、B同周期,两者相隔一元素;B、D同主族相邻,A、B、D三种元素的核电荷数之为和30。C、E能形成CE型离子化合物。F的次外层有8个电子,已知它的最外层不止一个电子。回答:
(1)写出各元素名称:A B C D E F
(2)A、D、E最高价氧化物的水化物酸性由强到弱的顺序(填化学式):
(3)比较A、B气态氢化物的稳定性(填化学式):
(4)比较D、E氢化物的还原性(填化学式):
(1)写出各元素名称:A B C D E F
(2)A、D、E最高价氧化物的水化物酸性由强到弱的顺序(填化学式):
(3)比较A、B气态氢化物的稳定性(填化学式):
(4)比较D、E氢化物的还原性(填化学式):

略

练习册系列答案
相关题目

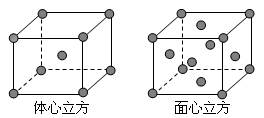

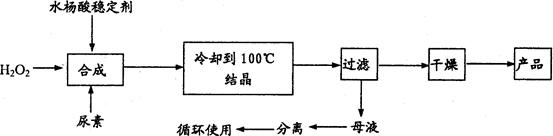
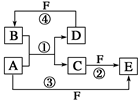
 ________________________________________。
________________________________________。 质,且反应①在水溶液中进行。
质,且反应①在水溶液中进行。