��Ŀ����
����������[CO��NH2��2��H2O2 ]��һ��������ζ�İ�ɫ�ᾧ��ĩ���������غ�������˫�����ʣ���һ�����͵������������������㷺Ӧ����Ư�ס���֯��ҽҩ��ũҵ����ֳҵ��������ϳ����£�
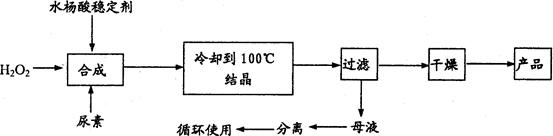
�Իش��������⣺
��1��ʵ�������������n��H2O2��:n��CO��NH2��2��]=1��2:1�������ƺϳ��¶���25��30�棬����Ҫԭ����___________________________________��
��2����ĸҺ�з����H2O2�����أ����õIJ�����___________��
��a������ ���� ��b����Һ ���� ��c����ѹ���� �ᾧ ��d����ѹ���� ��ȡ
��3��Ϊ�ⶨ��Ʒ�л������ĺ�����������16�����൱��H2O2 34��������ȡ������Ʒ12��000g���ܽ⣬��250mL����ƿ�ж��ݡ�ȷ��ȡ25��00mL����ƿ�У�����1mL 6mol��L�����ᣬȻ����0��2000 mol��L KMnO4����Һ�ζ������������һ��ʱ����Һ��dz��ɫ�Ұ�����ڲ���ɫ�����εζ�ƽ������KMnO4��Һ20��00mL��KMnO4��Һ�����ز���Ӧ����
��KMnO4��ҺӦʢ����________ʽ��ѡ����ᡱ��������ζ����С�
����ɲ���ƽ����ʽ��__MnO4-+ ___H2O2+___H+=___Mn2++ ____H2O+___ _____
�۸��ݵζ��������ȷ����Ʒ�л���������������Ϊ��___________��
�����ζ�ǰ�ζ��ܼ��촦�����ݣ��ζ�����ʧ����ʹ��õĻ���������______��ѡ���ƫ�ߡ�����ƫ�͡����䡱����
�ݸ��ݱ���ʵ���õĻ��������������жϸ�ʵ���Ʒ������һ����Ҫ����Ϊ_________��

�Իش��������⣺
��1��ʵ�������������n��H2O2��:n��CO��NH2��2��]=1��2:1�������ƺϳ��¶���25��30�棬����Ҫԭ����___________________________________��
��2����ĸҺ�з����H2O2�����أ����õIJ�����___________��
��a������ ���� ��b����Һ ���� ��c����ѹ���� �ᾧ ��d����ѹ���� ��ȡ
��3��Ϊ�ⶨ��Ʒ�л������ĺ�����������16�����൱��H2O2 34��������ȡ������Ʒ12��000g���ܽ⣬��250mL����ƿ�ж��ݡ�ȷ��ȡ25��00mL����ƿ�У�����1mL 6mol��L�����ᣬȻ����0��2000 mol��L KMnO4����Һ�ζ������������һ��ʱ����Һ��dz��ɫ�Ұ�����ڲ���ɫ�����εζ�ƽ������KMnO4��Һ20��00mL��KMnO4��Һ�����ز���Ӧ����
��KMnO4��ҺӦʢ����________ʽ��ѡ����ᡱ��������ζ����С�
����ɲ���ƽ����ʽ��__MnO4-+ ___H2O2+___H+=___Mn2++ ____H2O+___ _____
�۸��ݵζ��������ȷ����Ʒ�л���������������Ϊ��___________��
�����ζ�ǰ�ζ��ܼ��촦�����ݣ��ζ�����ʧ����ʹ��õĻ���������______��ѡ���ƫ�ߡ�����ƫ�͡����䡱����
�ݸ��ݱ���ʵ���õĻ��������������жϸ�ʵ���Ʒ������һ����Ҫ����Ϊ_________��
��1��H2O2��ʵ������л��в��ַֽ⣬������������������߹����� ���صĴ��ȣ�2�֣���
���صĴ��ȣ�2�֣���
��2��c��2�֣�
��3�����ᣨ2�֣�����2��5��6=2��8�� 5O2����2�֣���13��3����2�֣���
��ƫ�ߣ�2�֣� �����أ�дˮ�����ȶ���Ҳ�÷֣���2�֣�
 ���صĴ��ȣ�2�֣���
���صĴ��ȣ�2�֣�����2��c��2�֣�
��3�����ᣨ2�֣�����2��5��6=2��8�� 5O2����2�֣���13��3����2�֣���
��ƫ�ߣ�2�֣� �����أ�дˮ�����ȶ���Ҳ�÷֣���2�֣�
��

��ϰ��ϵ�д�
�����Ŀ

 ����ѧ����ʽ��
����ѧ����ʽ�� 
 ��
��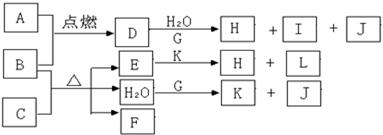

 д����Eת���F������ ���䷢����Ӧ�Ļ�ѧ����ʽ________��
д����Eת���F������ ���䷢����Ӧ�Ļ�ѧ����ʽ________�� B��C��D��ԭ��������С��18��A��Dͬ���壬B��C��ͬһ���ڣ�A��Dԭ�ӵ���������������1��Cԭ��������������Bԭ����2������C�����������Ǵ�����������2����A��B�����ڳ����¾�Ϊ���壬�����ڸ������������2��1��ȫ��Ӧ���������ڳ�������Һ�塣��Һ����D�����ܼ��ҷ�Ӧ����A�ĵ��ʡ�������Һ�����̪�Ժ�ɫ��ͬʱ��Һ�к�������ԭ�ӵĵ��Ӳ�ṹ��ͬ�������ӡ��ش��������⣺
B��C��D��ԭ��������С��18��A��Dͬ���壬B��C��ͬһ���ڣ�A��Dԭ�ӵ���������������1��Cԭ��������������Bԭ����2������C�����������Ǵ�����������2����A��B�����ڳ����¾�Ϊ���壬�����ڸ������������2��1��ȫ��Ӧ���������ڳ�������Һ�塣��Һ����D�����ܼ��ҷ�Ӧ����A�ĵ��ʡ�������Һ�����̪�Ժ�ɫ��ͬʱ��Һ�к�������ԭ�ӵĵ��Ӳ�ṹ��ͬ�������ӡ��ش��������⣺