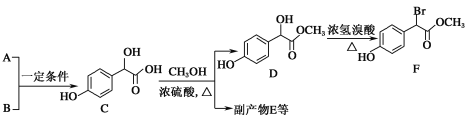
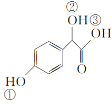
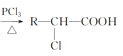��Ŀ����
����Ŀ��GaAs������۵�ܸ�, Ӳ�Ⱥܴ�, �ܶ�Ϊ�� g��cm-3,Ga ��As ��Ħ�������ֱ�ΪMGag��mol-1��MAs g��mol-1, ԭ�Ӱ뾶�ֱ�ΪrGa pm ��rAs pm, �����ӵ�����ֵΪNA, �侧���ṹ����ͼ��ʾ, ����˵���������
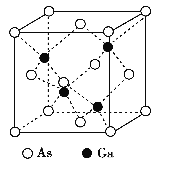
A. �þ���Ϊԭ�Ӿ���
B. �ڸþ�����,Ga��As���¶Ե���,Ga��As����λ����Ϊ4
C. ԭ�ӵ����ռ��������İٷ���Ϊ![]()
D. ����ԭ�Ӿ�����8�����ȶ��ṹ
���𰸡�C
��������
A��GaAs ������۵�ܸߣ�Ӳ�Ⱥܴ���ԭ�Ӿ�����ص���
B���ɾ����ṹ��֪��������As��Gaԭ����Ŀ֮��Ϊ1��1��As��Ga��λ����ͬ��Ga����Χ4��Asԭ���γ���������ṹ��As����Χ4��Gaԭ��Ҳ�γ���������ṹ��ԭ�Ӿ��γ�4������Gaԭ�Ӽ۵���Ϊ3����As�γ�4�����ۼ���˵��Asԭ���ṩ1�Թµ��ӶԸ�Ga�γ���λ����
C����̯�����㾧���и�ԭ����Ŀ���ټ���ԭ�������������ܶȼ��㾧��������ռ�������=�������ԭ�������/���������100%��
D��ÿ��As��Gaԭ�Ӷ��γ�4�����ۼ�����û�йµ��Ӷԡ�
A��GaAs ������۵�ܸߣ�Ӳ�Ⱥܴ�Ϊ�ռ�������վ�ṹ������ԭ�Ӿ��壬��A��ȷ��
B���ɾ����ṹ��֪��Ga����λ��Ϊ4��������Gaԭ����Ŀ=4��Asԭ����Ŀ=8��1/8+6��1/2=4��������As��Gaԭ����Ŀ֮��Ϊ1��1����As��λ��Ҳ��4��Ga����Χ4��Asԭ���γ���������ṹ��As����Χ4��Gaԭ��Ҳ�γ���������ṹ��ԭ�Ӿ��γ�4������Gaԭ�Ӽ۵���Ϊ3����As�γ�4�����ۼ���˵��Asԭ���ṩ1�Թµ��ӶԸ�Ga�γ���λ����Asԭ�������5������ȫ���ɼ�����û�йµ��Ӷԣ���B��ȷ��
C��������ԭ�������=4��4/3�У�rGa3+rAs3 ����10-30 cm3����������=4��(MGa+MAs)/NA g���������=��4��(MGa+MAs)/NA g���¦� g��cm-3��ԭ�ӵ����ռ��������İٷ���=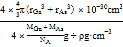 ��100%=4�Ц�NA(r3Ga+r3As)/3(MGa+MAs)��10-30��100%����C����
��100%=4��NA(r3Ga+r3As)/3(MGa+MAs)��10-30��100%����C����
D��ÿ��As��Gaԭ�Ӷ��γ�4�����ۼ�����û�йµ��Ӷԣ�����ԭ�Ӿ����� 8 �����ȶ��ṹ����D��ȷ��
��ѡ��C��

 �Ķ��쳵ϵ�д�
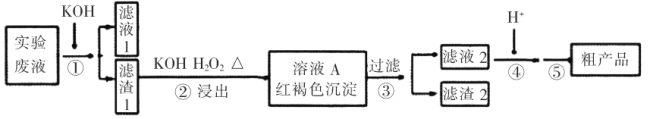
�Ķ��쳵ϵ�д�����Ŀ��ijʵ���ҷ�Һ��![]() �����ӣ���ͨ���������̱��Ϊ���Ʊ�
�����ӣ���ͨ���������̱��Ϊ���Ʊ�![]() ��
��
��֪��(a)![]()
![]() (��ɫ)
(��ɫ)
(b)����������������������pH�������
�������� | pH | |
���� | ���� | |
Fe3+ | 2.7 | 3.7 |
Cr3+ | 4.9 | 6.8 |
��ش�
(1)�����Թ��м�������(NH4)2Cr2O7���壬�μ�����ŨKOH��Һ�����ȣ��۲쵽����Ҫ�����ǣ�_________________��
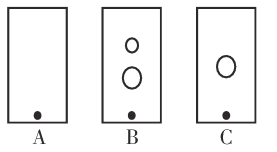
(2)ijͬѧ����ֽ�������жϲ���ټ���KOH�����Ƿ���ʡ��ڼ���һ����KOH��Һ��,��ëϸ��ȡ��������������ɫ��չ������Ѭ��ĵ���ͼ��ʾ������KOH���ʺϵ�ʵ������(��ͼABC��ѡ��ʵ��˳������)_________________,C�İߵ���ɫΪ_________________��
(3)����ں�Cr���ʷ�������Ҫ��Ӧ�����ӷ���ʽΪ_________________��
(4)������װ���У������Ӧѡ��ʵ��װ����_________________��(����)
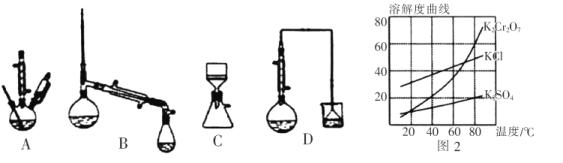
(5)�������ʵ��ܽ��������ͼ2,����ݿ����õ����в��ֲ���:a.���������ִ������壬ֹͣ����;b.��ȴ������;c.��������Һ���־�Ĥ��ֹͣ����;d.ϴ��;e.���ȹ���;f.���ˡ���ѡ����ʲ�������ȷ˳��_________________��
(6)������к��ʵ�ϴ�Ӽ���_________________(����ˮ�Ҵ��������Ҵ�-ˮ���Һ��������ˮ��������ˮ��),�ֲ�Ʒ��һ���ᴿ�ķ�����_________________��
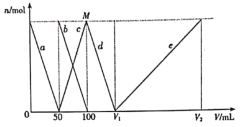
(7)ȡmg�ֲ�Ʒ���250mL��Һ��ȡ25.00mL����ƿ�У���cmol.L-1��![]() ����Һ�ζ�(���ʲ���Ӧ)�����ı�
����Һ�ζ�(���ʲ���Ӧ)�����ı�![]() ��ҺVmL����ôֲ�Ʒ��
��ҺVmL����ôֲ�Ʒ��![]() �Ĵ���Ϊ_________________��(
�Ĵ���Ϊ_________________��(![]() ��ʽ��:294)
��ʽ��:294)