��Ŀ����
����Ŀ������������������Ҫ��ҽҩ�м��塣��A��BΪԭ�Ϻϳɱ�����������F��·����ͼ��
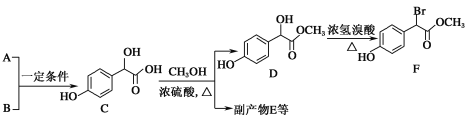
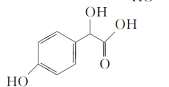
��1��A����ʽΪC2H2O3���ɷ���������Ӧ���Ҿ������ԣ�A��������������Ϊ__��B����ʽΪC6H6O��д��A��B��C�Ļ�ѧ��Ӧ����ʽ��__��
��2��C(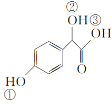 )�Т١��ڡ���3����OH��������ǿ������˳����__��
)�Т١��ڡ���3����OH��������ǿ������˳����__��
��3��1molF��һ��������������NaOH��Һ��Ӧ���������NaOH�����ʵ���Ϊ__mol����������������F��ͬ���칹��(�����������칹)����___�֡�
������̼��������Һ��Ӧ�ڱ�����ֻ��2��ȡ����������һ�����ǻ�
��4����֪��R��CH2��COOH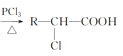 ��A�ж��ֺϳɷ������ڷ�����д��������ϳ�A��·������ͼ(����ԭ����ѡ)___��
��A�ж��ֺϳɷ������ڷ�����д��������ϳ�A��·������ͼ(����ԭ����ѡ)___��
�ϳ�·������ͼʾ�����£�H2C=CH2![]() CH3CH2OH
CH3CH2OH![]() CH3COOC2H5
CH3COOC2H5

���𰸡�ȩ�����Ȼ� 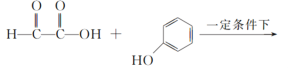
 ��>��>�� 3 12 CH3COOH
��>��>�� 3 12 CH3COOH
��������
A����ʽΪC2H2O3���ɷ���������Ӧ���Ҿ������ԣ���A��OHC-COOH������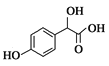 ���ƣ�B��
���ƣ�B��![]() ��
��
(1)A����ʽΪC2H2O3���ɷ���������Ӧ���Ҿ������ԣ�A��OHC-COOH����������������Ϊȩ�����Ȼ���B����ʽΪC6H6O������C���ƣ�B��![]() �� A��B��C�Ļ�ѧ��Ӧ����ʽ��
�� A��B��C�Ļ�ѧ��Ӧ����ʽ��
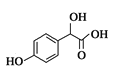 ��
��
(2)C(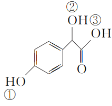 )�Т��Ƿ��ǻ������Ǵ��ǻ��������Ȼ���3����OH��������ǿ������˳���Ǣ�>��>�ڣ�
)�Т��Ƿ��ǻ������Ǵ��ǻ��������Ȼ���3����OH��������ǿ������˳���Ǣ�>��>�ڣ�
(3)1mol ��һ��������������NaOH��Һ��Ӧ����1mol
��һ��������������NaOH��Һ��Ӧ����1mol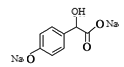 ��1molNaBr���������NaOH�����ʵ���Ϊ3mol��
��1molNaBr���������NaOH�����ʵ���Ϊ3mol��  ��ͬ���칹�壬������̼��������Һ��Ӧ˵�������Ȼ����ڱ�����ֻ��2��ȡ����������һ�����ǻ�������������2��ȡ�����ֱ���-OH��-CHBrCH2COOH��-OH��-CH2CHBrCOOH��-OH��-CBr(CH3)COOH��-OH��-CH(CH2Br)COOH��ÿ��������ڼ������λ���칹�����������Ľṹ��12�֣�
��ͬ���칹�壬������̼��������Һ��Ӧ˵�������Ȼ����ڱ�����ֻ��2��ȡ����������һ�����ǻ�������������2��ȡ�����ֱ���-OH��-CHBrCH2COOH��-OH��-CH2CHBrCOOH��-OH��-CBr(CH3)COOH��-OH��-CH(CH2Br)COOH��ÿ��������ڼ������λ���칹�����������Ľṹ��12�֣�
(4)������PCl3��������������![]() ��
��![]() ˮ��Ϊ
ˮ��Ϊ![]() ��
��![]() �ữ����
�ữ����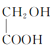 ��
��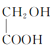 ����Ϊ
����Ϊ![]() ���ϳ�·������ΪCH3COOH
���ϳ�·������ΪCH3COOH ��
��

 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�����Ŀ���±��г���5��Ԫ�������ڱ��е�λ�á�
| ��A | 0 | ||||||
1 | ��A | ��A | ��A | ��A | ��A | ��A | ||
2 | �� | �� | ||||||
3 | �� | �� | �� |
(1)����Ԫ�ط�����________������ԭ�ṹʾ��ͼ��________________��
(2)����Ԫ���У���������ǿ����________________(��Ԫ�ط���)��
(3)��Ԫ�ص�����������Ӧ��ˮ�����________��(������������������������)��
(4)�ۢܢ�����Ԫ�ذ�ԭ�Ӱ뾶�ɴ�С��˳����__________(��Ԫ�ط���)��
(5)Ԫ������Ԫ�����γɵĻ������������________________��
(6)����Ԫ�����ĵ���ͨ��������________��(����ú��������ˮ��)��
(7)Ԫ������Ԫ�����ĵ��������Խ�ǿ����________(�ѧʽ)��
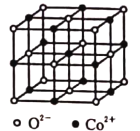
����Ŀ�������£��ú��ܷ���(��Ҫ�ɷ�ΪCoCO3������������NiCO3����м)�Ʊ�CoCl26H2O�Ĺ���������ͼ��
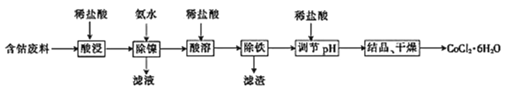
����֪������������ҺpH���ܵĻ����ʼ�Ni2+������Ӱ����ͼ��ʾ��
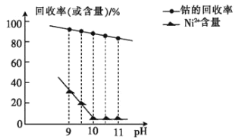
�ڲ��ֽ�����������ʵ�������¿�ʼ��������ȫ������pH�����ʾ��
���������� | ��ʼ����pH | ��ȫ����pH |
Fe3+ | 1.5 | 4.0 |
Fe2+ | 7.5 | 9.7 |
Co2+ | 6.6 | 9.4 |
Ni2+ | 7.7 | 9.5 |
�ش��������⣺
(1)�������Һ�е�������Ϊ��Co2+��Ni2+��__��
(2)����ʱ��Ӧ������ҺpH=__����ʱNi2+�Ƿ��γ�Ni(OH)2������___(�ǻ��)����pH��С�����Ʒ���Ȼ�___(���ߣ����ͣ���)��
(3)����ʱ��������pH=8ʱ����Һ��n(Fe3+)��n(Co2+)=__��
��֪Ksp[Co(OH)2]=2.0��10-16��Ksp[Fe(OH)3]=4.0��10-38��
(4)����ʱ������Һ�м���30%��H2O2���������ӷ�Ӧ����ʽ�ǣ�__����ַ�Ӧ��������Һ�м���CoCO3��������ҺpH��ΧΪ__��ʹFe3+��ȫת��ΪFe(OH)3�����������˵õ�CoCl2��Һ��
(5)��֪Ag++SCN-=AgSCN����Ϊ�ⶨ�ֲ�Ʒ��CoCl26H2O�ĺ�������ȡ11.9g�ֲ�Ʒ���100mL��Һ������ȡ��25mL�ȼ��뺬0.03mol��AgNO3��(���ʲ����䷴Ӧ)������0.5mol/L��KSCN��Һ�궨������AgNO3���ñ궨�������õ�ָʾ��Ϊ__(�ѧʽ)��������20.00mL��KSCN��Һ����ôֲ�Ʒ��CoCl26H2O����������Ϊ__��
����Ŀ��̼��������Ҫ�Ļ���ԭ��֮һ���㷺Ӧ�����Ṥ�ջ������ġ�ʳƷ��ҵ����ҵ��
(1)����̼�����к����Ȼ������ʣ�ѡ�������Լ����ʵ�鷽�����м��飬�Լ���ϡH2 SO4��BaCl2��Һ��Ca(NO3)2��Һ��AgNO3��Һ
ʵ�鲽�� | �� �� |
��ȡ������Ʒ��������ˮ�ܽ� | �ڹ�����ȫ�ܽ�õ���ɫ������Һ |
��_____ | ���а�ɫ�������� |
�ݾ��ã�________ | �� ________ |
(2)��AgNO3����Һ�ζ�Cl-(��K2CrO4��ҺΪָʾ����Ag2CrO4Ϊש��ɫ����)�����ⶨ̼���ƵĴ��ȡ�
��������Ʒ��Һ����ȡij̼������Ʒmg��������ƿ����100 mL��Һ������ʱ����____������ˮ���̶��ߡ�
������AgNO3��Һ��Ũ�ȱ궨����ȡAgNO3����8.5 g���ձ��У���____ȡ500 mLˮ�������ձ��У�����ҡ�Ⱥ�ת��____ɫ�Լ�ƿ�����ڰ��������á���NaCl����Һ�궨����AgNO3��ҺŨ��Ϊb mol/L��
�۵ζ���Ʒ��ȡ��Ʒ��Һ20. 00 mL����ƿ�У��ӹ���ϡHNO3������2��3��K2 CrO4��Һ����AgNO3����Һ�ζ����ζ��յ������Ϊ_________��
�ظ������������Ρ��Ĵβⶨ�������±���
ʵ����� | 1 | 2 | 3 | 4 |
����AgNO3��Һ���/mL | 20.00 | 21. 55 | 20. 02 | 19. 98 |
���ڵζ��յ��ȡ�ζ��̶ܿ�ʱ���ӱ�ҺҺ�棬��ⶨ���____(����ƫ��������ƫ����������Ӱ����)����Ʒ�Ĵ���Ϊ____%��