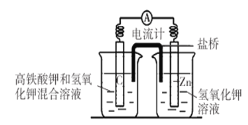
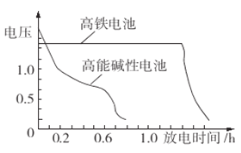
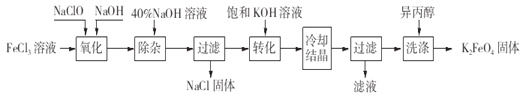
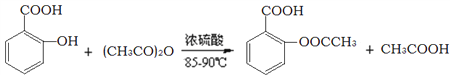
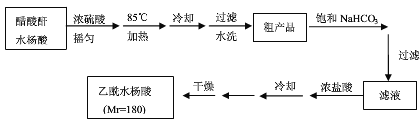
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ“―÷ΣΝΫΗωτ«ΜυΆ§ ±Ν§‘ΎΆ§“ΜΧΦ‘≠Ή”…œΒΡΫαΙΙ «≤ΜΈ»Ε®ΒΡΘ§Υϋ“ΣΖΔ…ζΆ―Υ°Ζ¥”ΠΘΚ

œ÷”–Ζ÷Ή” ΫΈΣC9H8O2X2Θ®XΈΣ“ΜΈ¥÷Σ‘ΣΥΊΘ©ΒΡΈο÷ MΘ§Ω…‘Ύ“ΜΕ®ΧθΦΰœ¬ΖΔ…ζ…œ ω“ΜœΒΝ–Ζ¥”Π
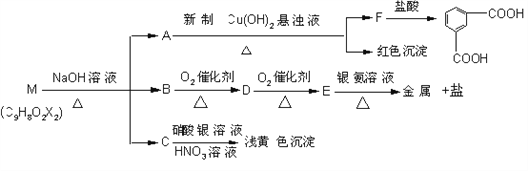
‘ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©XΈΣ____________________Θ®Χν‘ΣΥΊΖϊΚ≈Θ©ΘΜ
Θ®2Θ©…œ ωΉΣΜ·÷–Τδ÷– τ”Ύ―θΜ·Ζ¥”ΠΒΡΙ≤”–_______≤ΫΘ®Χν ΐΉ÷Θ©ΘΜM”κNaOH»ή“ΚΙ≤»»Ζ¥”ΠΒΡΥυ τάύ–Ά «_______________ Ζ¥”ΠΓΘ
Θ®3Θ©MΒΡΫαΙΙΦρ ΫΈΣ ________________________________
Θ®4Θ©–¥≥ωœ¬Ν–Ζ¥”ΠΒΡΜ·―ßΖΫ≥ΧΘΚ
EΚΆ“χΑ±»ή“ΚΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΘΚ_______________________________________________
ΓΨ¥πΑΗΓΩ Br 4 »Γ¥ζΘ®ΜρΥ°ΫβΘ© 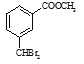 HCOOHΘΪ2Ag(NH3)2OH
HCOOHΘΪ2Ag(NH3)2OH ![]() (NH4)2CO3ΘΪ2AgΓΐΘΪ2NH3Γϋ+H2O
(NH4)2CO3ΘΪ2AgΓΐΘΪ2NH3Γϋ+H2O
ΓΨΫβΈωΓΩΗυΨί“―÷Σ–≈œΔΚΆMΒΡΉΣΜ·ΧθΦΰΘ§Ω…“‘ΆΤΕœ≥ωA÷–Κ§”–»©ΜυΘ§«“AΈΣτ»Υα―ΈΘ§BΈΣ¥ΦΓΘ”…”ΎBΨ≠ΝΫ≤Ϋ―θΜ·Ζ¥”ΠΒΟΒΫτ»ΥαEΘ§«“EΡήΖΔ…ζ“χΨΒΖ¥”ΠΘ§Υυ“‘EΈΣΦΉΥαΘ§‘ρBΈΣΦΉ¥ΦΘ§ΫχΕχΆΤΕœ≥ωM÷–¥φ‘Ύ“ΜΗωθΞΜυΓΘ”…C”κœθΥα“χ»ή“ΚΖ¥”Π…ζ≥…≤Μ»ή”ΎœΓœθΥαΒΡΒ≠ΜΤ…Ϊ≥ΝΒμΘ§ΆΤ÷ΣM÷–Κ§”–ΝΫΗωδε‘≠Ή”ΓΘ”…A±Μ–¬÷ΤCu(OH)2―θΜ·…ζ≥…FΘ§F”κ―ΈΥαΖ¥”ΠΥυΒΟ≤ζΈοΒΡΫαΙΙΩ…“‘ΆΤ≥ωΘ§AΓΔFΒΡΫαΙΙΦρ ΫΖ÷±πΈΣ![]() ΚΆ
ΚΆ![]() Θ§Ι MΈΣ
Θ§Ι MΈΣ![]() ΓΘ
ΓΘ
Θ®1Θ©ΫαΚœ“‘…œΖ÷ΈωΩ…÷ΣΘ§”…C”κœθΥα“χ»ή“ΚΖ¥”Π…ζ≥…≤Μ»ή”ΎœΓœθΥαΒΡΒ≠ΜΤ…Ϊ≥ΝΒμΘ§”ΠΈΣδεΜ·“χ≥ΝΒμΘ§Υυ“‘XΈΣBrΘΜ’ΐ»Ζ¥πΑΗΘΚBr ΓΘ
Θ®2Θ©”–ΜζΈοA±Μ–¬÷ΤCu(OH)2―θΜ·…ζ≥…FΘ§”–ΜζΈοB±Μ―θΜ·ΈΣDΘ§D”–±Μ―θΜ·ΈΣE, E”÷±Μ“χΑ±»ή“Κ―θΜ·Θ§Υυ“‘…œ ωΉΣΜ·÷– τ”Ύ―θΜ·Ζ¥”ΠΒΡΙ≤”–4≤ΫΘΜ”–ΜζΈοMΒΡΫαΙΙΦρ ΫΈΣ![]() Θ§Υϋ”κNaOH»ή“ΚΙ≤»»Ζ¥ΖΔ…ζΝΥΥ°ΫβΖ¥”ΠΘΜ’ΐ»Ζ¥πΑΗΘΚ4ΘΜ»Γ¥ζΘ®ΜρΥ°ΫβΘ©ΓΘ
Θ§Υϋ”κNaOH»ή“ΚΙ≤»»Ζ¥ΖΔ…ζΝΥΥ°ΫβΖ¥”ΠΘΜ’ΐ»Ζ¥πΑΗΘΚ4ΘΜ»Γ¥ζΘ®ΜρΥ°ΫβΘ©ΓΘ
Θ®3Θ©ΗυΨί“‘…œΖ÷ΈωΩ…÷ΣΘΚMΒΡΫαΙΙΦρ ΫΈΣ ΘΜ’ΐ»Ζ¥πΑΗΘΚ
ΘΜ’ΐ»Ζ¥πΑΗΘΚ ΓΘ
ΓΘ
Θ®4Θ©”–ΜζΈοEΈΣHCOOHΘ§Κ§”–»©ΜυΘ§Ω…±Μ“χΑ±»ή“Κ―θΜ·Θ§Ζ¥”ΠΒΡΜ·―ßΖΫ≥ΧΈΣΘΚHCOOHΘΪ2Ag(NH3)2OH ![]() (NH4)2CO3ΘΪ2AgΓΐΘΪ2NH3Γϋ+H2OΘΜ’ΐ»Ζ¥πΑΗΘΚHCOOHΘΪ2Ag(NH3)2OH
(NH4)2CO3ΘΪ2AgΓΐΘΪ2NH3Γϋ+H2OΘΜ’ΐ»Ζ¥πΑΗΘΚHCOOHΘΪ2Ag(NH3)2OH ![]() (NH4)2CO3ΘΪ2AgΓΐΘΪ2NH3Γϋ+H2OΓΘ
(NH4)2CO3ΘΪ2AgΓΐΘΪ2NH3Γϋ+H2OΓΘ

 ‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ
‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗΓΨΧβΡΩΓΩΘ®1Θ©AΓΔBΓΔCΓΔDΈΣ÷–―ß≥ΘΦϊΒΡΜλΚœΈοΖ÷άκΜρΧα¥ΩΒΡΜυ±ΨΉΑ÷ΟΘ§«κΜΊ¥πΘΚ
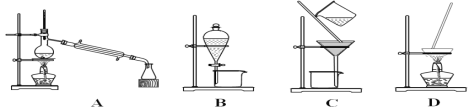
ΔΌΖ÷άκ÷≤Έο”ΆΚΆΥ°Θ§―Γ‘ώΉΑ÷Ο______Θ®Χν–ρΚ≈Θ©Θ§÷ς“Σ Ι”Ο“«ΤςΟϊ≥Τ______ΘΜ
ΔΎΖ÷άκ““Εΰ¥ΦΚΆ±ϊ»ΐ¥ΦΒΡΜλΚœ»ή“ΚΘ§―Γ‘ώΉΑ÷Ο______Θ®Χν–ρΚ≈Θ©ΓΘ
Έο÷ | »έΒψ Θ®…ψ œΕ»Θ© | Ζ–ΒψΘ®…ψ œΕ»Θ© | ΟήΕ»Θ®g/cm-3Θ© | »ήΫβ–‘ |
““Εΰ¥Φ | -11.5 | 198 | 1.11 | “Ή»ή”ΎΥ°ΚΆ““¥Φ |
±ϊ»ΐ¥Φ | 17.9 | 290 | 1.26 | ΡήΗζΥ°ΓΔΨΤΨΪ“‘»Έ“β±»άΐΜΞ»ή |
Θ®2Θ© Β―ι “–η≈δ÷Τ250mL0.1molΓΛL-1ΒΡNa2CO3»ή“ΚΘ§ΧνΩ’≤ΔΜΊ¥πœ¬Ν–Έ ΧβΘΚ
ΔΌ≈δ÷Τ250mL0.1molΓΛL-1ΒΡNa2CO3»ή“ΚΘ§ ΒΦ ”Π≥Τ»ΓNa2CO3ΒΡ÷ ΝΩ «______gΘ§ Β―ιΙΐ≥Χ÷–Υυ–ηΒΡ≤ΘΝß“«Τς”–______ΓΘ
ΔΎ»τ‘Ύ»ή“Κ≈δ÷ΤΙΐ≥Χ÷–≥ωœ÷»γœ¬«ιΩωΘ§Ε‘Υυ≈δ»ή“Κ≈®Ε»ΫΪ”–ΚΈ”ΑœλΘ®ΧνΓΑΤΪΗΏΓ±ΓΑΤΪΒΆΓ±ΜρΓΑΈό”ΑœλΓ±Θ©
»τ»ίΝΩΤΩ÷–”–…ΌΝΩ’τΝσΥ°__________ΘΜ»τΕ®»ί ±Η© ”ΩΧΕ»œΏ__________ΓΘ
Θ®3Θ©ΫΙΧΩ”κ≈®ΝρΥαΦ”»»ΧθΦΰœ¬ΖΔ…ζ»γœ¬Ζ¥”ΠΘΚC +2H2 SO4Θ®≈®Θ©=2SO2Γϋ+ CO2Γϋ +2H2OΘ§Β±Ζ¥”Π÷–ΉΣ“Τ2moleΓΣ ±Θ§…ζ≥…SO2ΒΡΧεΜΐΘ®±ξΉΦΉ¥ΩωΘ© «____LΓΘ