��Ŀ����
5������������ȷ���ǣ�������| A�� | �����Ǵ�ˮ�������ԡ����Ի�����ϡ��Һ�������£���c��H+��•c��OH-��=1��10-14 | |
| B�� | ij��Һ��ˮ�������c��OH-��=10-a mol/L����a��7ʱ�������Һ��pHһ��Ϊ14-a | |
| C�� | 0.2 mol/L CH3COOH��Һ�е�c��H+����0.1 mol•L-1 CH3COOH��Һ�е�c��H+����2�� | |
| D�� | �κ�Ũ�ȵ���Һ��������pH����ʾ�����Ե�ǿ�� |
���� A��ˮ�����ӻ�ֻ���¶��йأ�
B����ˮ�������c��H+��=l��10-amol•L-1������Һ������������Һ��Ҳ�����Ǽ�����Һ���ݴ˼����жϣ�ע��Ϊ������Һʱ����Һ������������Ũ�ȵ���ˮ�����������Ũ�ȣ�����ˮ�����ӻ�������Һ�������ӣ��ٸ���pH=-logc��H+������pHֵ��
C�����ݴ���Ũ��������̶ȼ�С���з�����
D��������Һ��������PH����ʾ���䷶Χһ���� 0��14֮������жϣ�
��� �⣺A��ˮ�����ӻ�ֻ���¶��йأ����Գ�����Kw=c��H+��•c��OH-��=1��10-14������Һ��������أ���A��ȷ��
B����ˮ�������c��H+��=l��10-amol•L-1������Һ��Ϊ������Һ������Һ��c��OH-��=l��10-amol•L-1������Һ��c��H+��=$\frac{1{0}^{-14}}{1{0}^{-a}}$mol/L=l��10-14+amol/L����pH=-logl��10-14+a=14-a������Һ��Ϊ������Һ������ҺpH=-lgl��10-a=a����B����
C��������������ʣ�Ũ��������ĵ���̶ȼ�С������0.2 mol/L CH3COOH��Һ�е�c��H+��С��0.1 mol/L CH3COOH��Һ�е�c��H+����2������C����
D��pH��Χһ���� 0��14֮�䣬��Һ�е���������1��10-14mol/L����D����
��ѡA��
���� ���⿼����ˮ�ĵ���ƽ�⼰pH��ʹ�÷�Χ��ע���˻���֪ʶ�Ŀ��飬���Ը�����ѧ֪ʶ��ɣ������ѶȲ���

| A�� | X2Y3 | B�� | X3Y2 | C�� | Y2X3 | D�� | Y3X2 |
| A�� | 0.45 mol/L | B�� | 0.6 mol/L | C�� | 0.9 mol/L | D�� | 1.2 mol/L |
| A�� | ��ȥҺ������������ˮ���������м�����KI | |
| B�� | ��ˮ����������ʹʪ�����ɫ������ɫ������HClO���õĽ�� | |
| C�� | ij��ɫ������NaOHŨ��Һ��ϣ�����ʹʪ���ɫʯ����ֽ�����������������þ���һ����NH4Cl | |
| D�� | ij��Һ��ʹ���۵⻯����ֽ����������Һ��һ������Cl2 |
| A�� | ����Ϊ��̿��������O2 | B�� | ����ΪSO2��������NaOH��Һ | ||
| C�� | ����ΪFe������������ | D�� | ����ΪNaOH��Һ��������CO2 |
| A�� | 20% | B�� | 40% | C�� | 60% | D�� | 80% |
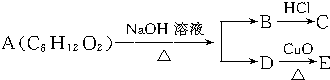
| A�� | 4�� | B�� | 3�� | C�� | 2�� | D�� | 1�� |
| A�� | �����ԭ�������� | B�� | �����������ɱ� | ||
| C�� | �����˻�����Ⱦ | D�� | �����˶��豸�ĸ�ʴ |