��Ŀ����
����Ŀ����ú��ʯ���п�������������ԭ��A��B��A��һ�ֹ�ʵ����������IJ�����������һ�����ҵ�ʯ�ͻ�����չˮƽ��B��һ�ֱ�ˮ�����״Һ̬����0.1mol��������������������ȫȼ�գ�����0.6molCO2��0.3molˮ���ش��������⣺
(1)A �ĵ���ʽΪ_____��B �Ľṹ��ʽΪ______��
(2)��A ���ڵ�ͬϵ��Cʹ������Ȼ�̼��Һ��ɫ�Ļ�ѧ��Ӧ����ʽ��____����Ӧ���ͣ�____��
(3)�ڵ�ˮ�м���B���ʣ���������ú������_________��
(4)B��Ũ�����Ũ������55��60�������·�Ӧ�Ļ�ѧ����ʽ��_______����Ӧ���ͣ�______��
(5)��������A��B��ȫȼ��ʱ����O2�����ʵ�����_______(����A>B����A<B������A��B��)��
���𰸡�![]()
![]() CH2=CHCH3+Br2��CH2BrCHBrCH3 �ӳɷ�Ӧ ��Һ�ֲ㣬�²���ɫ���ϲ���Ϻ�ɫ
CH2=CHCH3+Br2��CH2BrCHBrCH3 �ӳɷ�Ӧ ��Һ�ֲ㣬�²���ɫ���ϲ���Ϻ�ɫ ![]() +HO-NO2(Ũ)
+HO-NO2(Ũ)![]()
![]() +H2O ȡ����Ӧ A>B
+H2O ȡ����Ӧ A>B
��������
A��һ�ֹ�ʵ����������IJ�����������һ�����ҵ�ʯ�ͻ�����չˮƽ��AΪ��ϩ��B��һ�ֱ�ˮ�����״Һ̬����0.1mol��������������������ȫȼ�գ�����0.6molCO2��0.3molˮ����0.1molB����0.6molC��0.6molH��B�ķ���ʽΪC6H6��
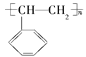
(1)AΪ��ϩ��C��H֮���ǵ�����C��C֮����˫���������ʽΪ![]() ��B�ķ���ʽΪC6H6����ṹ��ʽΪ
��B�ķ���ʽΪC6H6����ṹ��ʽΪ![]() ��
��
(2)AΪ��ϩ����A���ڵ�ͬϵ��Ϊ��ϩ���ṹ��ʽΪCH3CH=CH2�����巢���ӳɣ��仯ѧ����ʽΪCH2=CHCH3+Br2��CH2BrCHBrCH3��Ϊ�ӳɷ�Ӧ��
(3)���ܹ���ȡ��ˮ�еĵ⣬�����ܶ�С��ˮ���л������ϲ㣬�����ڱ��У����Ϻ�ɫ������Ϊ��Һ�ֲ㣬�²���ɫ���ϲ���Ϻ�ɫ��
(4)����Ũ���ᣬ��Ũ�����������������·���������Ӧ����������������ѧ����ʽΪ![]() +HO-NO2(Ũ)
+HO-NO2(Ũ)![]()
![]() +H2O����NO2ȡ���˱����ϵ�H��Ϊȡ����Ӧ��
+H2O����NO2ȡ���˱����ϵ�H��Ϊȡ����Ӧ��
(5)12gC��ȫȼ������1molO2��12gH2��ȫȼ������3molO2����֪��ͬ��������£�������Խ�ߣ�������Խ�ߡ���ϩ������ʽC2H4���京����Ϊ![]() ����������ʽΪC6H6���京����Ϊ
����������ʽΪC6H6���京����Ϊ![]() ����ϩ�ĺ��������ڱ��ĺ����������������A(��ϩ)��B(��)��ȫȼ��ʱ����O2�����ʵ�����A>B��
����ϩ�ĺ��������ڱ��ĺ����������������A(��ϩ)��B(��)��ȫȼ��ʱ����O2�����ʵ�����A>B��

����Ŀ������һ����ҽ�ÿ��ֵ�ԭ��֮һ��ϩ������ϳɲ��ϵĻ���ԭ�ϣ�����������Ϊһ��������ϩ�ķǻ�ʯȼ��·�߾��м�����Ҫ����ʵ���塣�������⼼����Ҫ��Ϊֱ������������������֡�
(1)�����±��ṩ�����ݣ��������ֱ�������Ʊ�ϩ�ķ�ӦC3H8(g)![]() C3H6(g) +H2(g)��H=___��
C3H6(g) +H2(g)��H=___��
���ۼ� | C-C | C=C | C-H | H-H |
����/(kJmol-1) | 348 | 615 | 413 | 436 |
(2)��ͼΪ����ֱ�������Ʊ�ϩ��Ӧ�б���ͱ�ϩ��ƽ������������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ѹǿ�ֱ�Ϊ1��104Pa��1��105Pa��

���ں����ܱ������У����������˵���÷�Ӧ�ﵽƽ��״̬����__������ĸ����
A��H���ֲ���
B�����������ܶȱ��ֲ���
C����������ƽ��Ħ���������ֲ���
D����λʱ��������1molH-H����ͬʱ����1molC=C��
����ʹ��ϩ��ƽ�������ߣ����д�ʩ���е���____������ĸ��
A������ѹǿ B�������¶� C�������ݻ�����������
��ҵ������Ϊ��߱�ϩ�IJ��ʣ������ں�ѹʱ��ԭ�����в���ˮ��������Ŀ����_____��
��1��104Paʱ��ͼ�б�ʾ����ͱ�ϩ������������߷ֱ���___��____�����ţ�
��1��104Pa��500��ʱ���÷�Ӧ��ƽ�ⳣ��Kp=____Pa����ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ=��ѹ�����ʵ���������������������λ��Ч���֣�
(3)����CO2���������ԣ���ѧ�ҿ����˱������������Ʊ�ϩ���¹��գ��ù��տɲ��ø�������������������֪C3H8+CO2(g) C3H6(g)+CO(g)+H2O(l)���ù��տ�����Ч������������Ļ�̿��ά�ִ����Ļ��ԣ���ԭ����____������ڱ���ֱ���ѽ������Ʊ�ϩ��ȱ����_____��
C3H6(g)+CO(g)+H2O(l)���ù��տ�����Ч������������Ļ�̿��ά�ִ����Ļ��ԣ���ԭ����____������ڱ���ֱ���ѽ������Ʊ�ϩ��ȱ����_____��