��Ŀ����
4�����г���ǰ20��Ԫ���е�ijЩԪ�����ʵ��й����ݣ�| Ԫ�ر�� Ԫ������ | �� | �� | �� | �� | �� | �� | �� | �� | �� | �� |
| ԭ�Ӱ뾶��10-10m�� | 1.52 | 2.27 | 0.74 | 1.43 | 0.77 | 1.10 | 0.99 | 1.86 | 0.75 | 0.71 |
| ���̬ | +1 | +1 | - | +3 | +4 | +5 | +7 | +1 | +5 | - |
| ��ͼ�̬ | - | - | -2 | - | -4 | -3 | -1 | - | -3 | -1 |
��2���������Ԫ�����ڱ��У���б�߱��ͨ��Ѱ�Ҵ������ϵ�����
��3������10��Ԫ�ص�ԭ���У�������ʧȥ���ӵ���K����Ԫ�ط��ţ�����H2�������ϵķǽ��������Ƿ�����д�������ƣ���
��4���õ���ʽ��ʾԪ�آ��γɵ��⻯�����
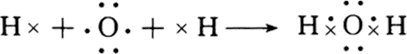
��5��д�������ݡ��ޡ�������Ԫ���е�ij����Ԫ���γɵĻ������У�ÿ��ԭ�Ӷ����������Ϊ8�����ȶ��ṹ��һ�����ʵķ���ʽCCl4��PCl3��ijԪ��R��ԭ�Ӱ뾶Ϊ1.02��10-10m����Ԫ�������ڱ���λ�ڵ������ڵ�VIA�壮
��6��д���ܵ�����������ˮ����ֱ���ߺ͢������������ˮ���ﷴӦ�����ӷ���ʽ��
���� �٢ڢ��������Ϊ+1��Ϊ��IA��Ԫ�أ�ԭ�Ӱ뾶�����ڢ�ԭ�Ӱ뾶�����ԭ�Ӱ뾶��֪������LiԪ�ء�����KԪ�ء�����NaԪ�أ�
�ߢ���ͼ�Ϊ-1��Ϊ��VIIA��Ԫ�أ��ߵ�ԭ�Ӱ뾶���ڢ⣬���Ԣ���ClԪ�ء�����FԪ�أ�
��ֻ��-2�ۣ�û�������ϼۣ�Ϊ��VIA��Ԫ�أ������OԪ�أ�
��ֻ��+3�ۣ�Ϊ��IIIA��Ԫ�أ�ԭ�Ӱ뾶����Clԭ�Ӱ뾶�����Ԣ���AlԪ�أ�
����+4��-4�ۣ�Ϊ��IVA��Ԫ�أ�ԭ�Ӱ뾶С��Clԭ�Ӱ뾶�����Ԣ���CԪ�أ�
�ޢ���+5��-3�ۣ�Ϊ��VA��Ԫ�أ���ԭ�Ӱ뾶���ڢᣬ�����PԪ�ء�����NԪ�أ�
������ʵ����ʷ�����ɣ�
��� �⣺�٢ڢ��������Ϊ+1��Ϊ��IA��Ԫ�أ�ԭ�Ӱ뾶�����ڢ�ԭ�Ӱ뾶�����ԭ�Ӱ뾶��֪������LiԪ�ء�����KԪ�ء�����NaԪ�أ�
�ߢ���ͼ�Ϊ-1��Ϊ��VIIA��Ԫ�أ��ߵ�ԭ�Ӱ뾶���ڢ⣬���Ԣ���ClԪ�ء�����FԪ�أ�
��ֻ��-2�ۣ�û�������ϼۣ�Ϊ��VIA��Ԫ�أ������OԪ�أ�
��ֻ��+3�ۣ�Ϊ��IIIA��Ԫ�أ�ԭ�Ӱ뾶����Clԭ�Ӱ뾶�����Ԣ���AlԪ�أ�
����+4��-4�ۣ�Ϊ��IVA��Ԫ�أ�ԭ�Ӱ뾶С��Clԭ�Ӱ뾶�����Ԣ���CԪ�أ�
�ޢ���+5��-3�ۣ�Ϊ��VA��Ԫ�أ���ԭ�Ӱ뾶���ڢᣬ�����PԪ�ء�����NԪ�أ�
��1����10��Ԫ��������Ԫ�����ڱ���λ�����£�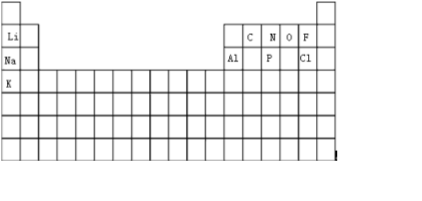 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2��ͨ���������IJ����ڹ���Ԫ�����ң�������Ϊ��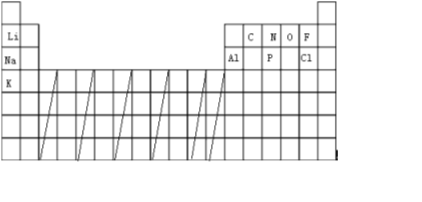 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��3�����Ӳ���Խ�࣬����������Խ�٣���ԭ�Ӻ˵�������ԽС��Խ����ʧȥ�������ӣ�����ЩԪ����K���Ӳ�����࣬��������������Ϊ1��Ԫ�صķǽ�����Խǿ������������Խ���ף�F�ķǽ�������ǿ���ʴ�Ϊ��K��������
��4��Ԫ�آ�ΪO��O�γɵ��⻯��Ϊˮ��H2OΪ���ۻ������ԭ�Ӵﵽ�ȶ��ṹ���õ���ʽ��ʾ�γɹ���Ϊ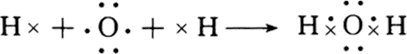 ���ʴ�Ϊ��
���ʴ�Ϊ��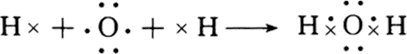 ����5��C��P��Cl����Ԫ���е�ij����Ԫ���γɵĻ�����������������8�����ӵ��ȶ��ṹ����CCl4��PCl3�ܹ�����ÿ��ԭ�ӵ������Ϊ8�����ȶ��ṹ��ԭ�Ӱ뾶Ϊ1.02��10-10m����ClȴС��P����RӦ��S�����ڵ������ڵ�VIA�壬�ʴ�Ϊ��CCl4��PCl3���������ڵ�VIA�壻
����5��C��P��Cl����Ԫ���е�ij����Ԫ���γɵĻ�����������������8�����ӵ��ȶ��ṹ����CCl4��PCl3�ܹ�����ÿ��ԭ�ӵ������Ϊ8�����ȶ��ṹ��ԭ�Ӱ뾶Ϊ1.02��10-10m����ClȴС��P����RӦ��S�����ڵ������ڵ�VIA�壬�ʴ�Ϊ��CCl4��PCl3���������ڵ�VIA�壻
��6����������Ϊ����ǿ����������������Ʒ�Ӧ����ƫ�����ƺ�ˮ��������ᷴӦ�������κ�ˮ�����ӷ�Ӧ����ʽ�ֱ�Ϊ��OH-+Al��OH��3=AlO2-+2H2O��Al��OH��3+3H+=Al3++3H2O���ʴ�Ϊ��OH-+Al��OH��3=AlO2-+2H2O��Al��OH��3+3H+=Al3++3H2O��
���� ���⿼��λ�á��ṹ�����ʵĹ�ϵ����������֪��Ϣ�Ƴ�Ԫ���ǽ����Ĺؼ��������ó���Ԫ�ء����ʵ����ʼ��ṹ����ɣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | XYZ2 | B�� | X2YZ3 | C�� | X2YZ2 | D�� | XYZ3 |
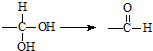 �����
���л���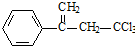 ��ѡ�������������Գ��ݼ����׳ư�ϩ����Ҫ����ˮ��������ݣ��¶Ⱥ�ʪ�ȶ�ҩЧӰ����¶ȸߡ�ʪ�ȴ�ҩЧ���ӿ죮�����йظ��л����˵������ȷ���ǣ�������
��ѡ�������������Գ��ݼ����׳ư�ϩ����Ҫ����ˮ��������ݣ��¶Ⱥ�ʪ�ȶ�ҩЧӰ����¶ȸߡ�ʪ�ȴ�ҩЧ���ӿ죮�����йظ��л����˵������ȷ���ǣ�������| A�� | ����±��������ʹ���Ը��������Һ����ˮ��ɫ | |
| B�� | �����ʼ��ж�ӳ�칹��Ҳ��˳���칹 | |
| C�� | �ڼ��������³��ˮ�⣬������������ | |
| D�� | 1mol ��������һ�������¿���4molH2�����ӳɷ�Ӧ |
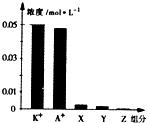 �����£�0.1mol•L-1һԪ��HA���Ũ��KOH��Һ�������Ϻ�������Һ�в�������ּ�Ũ����ͼ��ʾ������˵����ȷ���ǣ�������
�����£�0.1mol•L-1һԪ��HA���Ũ��KOH��Һ�������Ϻ�������Һ�в�������ּ�Ũ����ͼ��ʾ������˵����ȷ���ǣ�������| A�� | �û����ҺpH=7.0 | B�� | ԭHA��Һ�У�c��HA����c��H+����c��A-�� | ||
| C�� | ͼ��X��ʾHA��Y��ʾOH-��Z��ʾH+ | D�� | �����Һ�У�c��X��+x��A-��=c��K+�� |
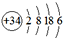 ��Ԫ�أ�λ��Ԫ�����ڱ��ģ�������
��Ԫ�أ�λ��Ԫ�����ڱ��ģ�������| A�� | �������ڵڢ��� | B�� | �������ڵڢ��� | C�� | �������ڵڢ�A�� | D�� | �������ڵڢ�A�� |
| A�� | 8 | B�� | 10 | C�� | 12 | D�� | 14 |
| A�� | ���Ƿ�������ԭ��Ӧ��������������ԭ��Ӧ | |
| B�� | ������Ӧ��SO2������ֻ�൱�ڴ��� | |
| C�� | ��Ӧ����CuSO4���������� | |
| D�� | ��Ӧ����SO2������ԭ��Ӧ |

 �ķ���ʽΪC12H16O��
�ķ���ʽΪC12H16O�� �����У�����ͬһƽ���ϵ�ԭ������������16����
�����У�����ͬһƽ���ϵ�ԭ������������16���� ϵͳ����Ϊ4��4-����-2-�촼��
ϵͳ����Ϊ4��4-����-2-�촼��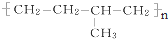 �ĵ���ΪCH2=CH2��CH3-CH=CH2��
�ĵ���ΪCH2=CH2��CH3-CH=CH2��