��Ŀ����
����Ŀ����ҵ�ϳ�ͨ�����·ֽ�FeSO4�ķ����Ʊ�Fe2O3���仯ѧ����ʽΪ��2FeSO4![]() Fe2O3��SO2����SO3����Ϊ����FeSO4���·ֽ�IJ����������ʵ�飺
Fe2O3��SO2����SO3����Ϊ����FeSO4���·ֽ�IJ����������ʵ�飺
��ȡ����FeSO4���·ֽ�õ��Ĺ��壬��һ����ϡ�����ܽ⣬�����Һ�м���������KSCN��Һ���۲���Һ��ɫ�ı仯�Լ���Fe3���Ƿ���ڡ�
�ڽ�FeSO4���·ֽ����������ͨ����ͼ��ʾ��װ���У��Լ���������������Ƿ���SO2��SO3��
��ش��������⣺
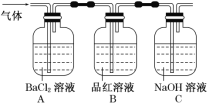
��1��д��KSCN��Һ��Fe3����Ӧ�����ӷ���ʽ��____��
��2���������й۲쵽������ͽ�����____��_____��
��3�������������Ҫ��ͨ��Ʒ����Һ������ͨ��NaOH��Һ�е�ԭ����___���йط�Ӧ�����ӷ���ʽ____��
���𰸡�Fe3����3SCN�� = Fe(SCN)3 Aƿ��Һ�г��ְ�ɫ������˵�������к���SO3 Bƿ��Һ�ɺ�ɫ��Ϊ��ɫ��˵�������к���SO2 ���ն����SO2��ֹ��Ⱦ���� SO2��2OH�� = SO32����H2O��SO32����SO2��H2O = 2HSO3��(���Բ�д)
��������
��KSCN��Һ��Fe3����Ӧ����Ѫ��ɫFe(SCN)3��
�Ʋ������������к���SO3����ʹAƿ��Һ���ְ�ɫ�����������к���SO2����ʹBƿ��Һ�ɺ�ɫ��Ϊ��ɫ��
�Dz����������Ҫ��ͨ��Ʒ����Һ������ʣ���������NaOH��Һ���������ն����SO2��ֹ��Ⱦ������
��KSCN��Һ��Fe3����Ӧ�����ӷ���ʽΪ��Fe3����3SCN��= Fe(SCN)3��
��������������ˮ�������ᣬ��������й۲쵽������ͽ�����Aƿ��Һ�г��ְ�ɫ������˵�������к���SO3��Bƿ��Һ�ɺ�ɫ��Ϊ��ɫ��˵�������к���SO2���ʴ�Ϊ��Aƿ��Һ�г��ְ�ɫ������˵�������к���SO3��Bƿ��Һ�ɺ�ɫ��Ϊ��ɫ��˵�������к���SO2��
�Ƕ��������Ǵ�����Ⱦ���˲����������Ҫ��ͨ��Ʒ����Һ������ͨ��NaOH��Һ�е�ԭ�������ն����SO2��ֹ��Ⱦ�������йط�Ӧ�����ӷ���ʽΪSO2��2OH��= SO32����H2O��SO32����SO2��H2O = 2HSO3��(���Բ�д)���ʴ�Ϊ�����ն����SO2��ֹ��Ⱦ������SO2��2OH��= SO32����H2O��SO32����SO2��H2O = 2HSO3��(���Բ�д)��

����Ŀ�����к͵ζ����ⶨij�ռ���Ʒ�Ĵ��ȣ��Ը���ʵ��ش��������⣺
��1��ȷ����8��2g�������������������ʵ���Ʒ�����500mL������Һ�� ��0��100mol![]() L-1����ζ���д�����кͷ�Ӧ���Ȼ�ѧ����ʽ____________(�к�����H����57��3 kJ/mol)�������ռ���Ʒ���500mL������Һ��Ҫ�IJ���������_________________________��
L-1����ζ���д�����кͷ�Ӧ���Ȼ�ѧ����ʽ____________(�к�����H����57��3 kJ/mol)�������ռ���Ʒ���500mL������Һ��Ҫ�IJ���������_________________________��
��3���ζ������У��۾�Ӧע��_________________�����÷�̪��ָʾ���ﵽ�ζ��յ���ɫ�仯��____________________________________��
��4�������±����ݣ����㱻���ռ���Һ�����ʵ���Ũ����_____molL-1���ռ���Ʒ�Ĵ�����____������С�������λ��
�ζ����� | ������Һ �����mL�� | ������� | |
�ζ�ǰ�Ŀ̶ȣ�mL�� | �ζ���Ŀ̶ȣ�mL�� | ||
��һ�� | 10��00 | 0��40 | 20��50 |
�ڶ��� | 10��00 | 4��10 | 24��00 |
��5������ʵ�������Եζ��������ʲô�����������ƫ������ƫ����������Ӱ������
�� �۲���ʽ�ζ���Һ��ʱ����ʼ���ӣ��ζ��յ�ƽ�ӣ���ζ����____��
�� ������ƿ�ô���Һ��ϴ��Ȼ���ټ���10��00mL����Һ����ζ����______________��