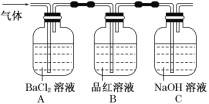
��Ŀ����
����Ŀ����֪п��Ũ���ᷴӦ����SO2����ϡ���ᷴӦ����H2��ʹһ������п��100mL18.5mol��L��1Ũ�����ַ�Ӧ��п��ȫ�ܽ⣬ͬʱ�ռ�����״���µ�����A33.6L������Ӧ�����Һϡ�͵�1L�������Һ�������ӵ�Ũ��Ϊ0.1mol��L��1��
��1��д��п��Ũ���ᷴӦ�Ļ�ѧ����ʽ��____��
��2����Ӧ���������ĵ�H2SO4�����ʵ�����____��
��3������A�ijɷ���____�����ɷֵ��������____��
��4����Ӧ���������ĵ�п��������____��
���𰸡�Zn��2H2SO4(Ũ)=ZnSO4��SO2����2H2O 1.80mol ������������� 1��4 97.5g
��������
��п��Ũ���ᷴӦ��������п�����������ˮ��
�Ʒ�Ӧ����������H2SO4�����ʵ��������ܵ������ȥʣ������ᡣ
����������A�����ʵ������ٸ��������ü���˼ά�ж��Ƿ�Ϊ��һ���壬���ݶ���������������ʵ������غ��ϵ������ϵʽ���㡣
�ȸ���������ѧ����ʽ��֪n(Zn)��n(SO2)��n(H2)���㡣
��п��Ũ���ᷴӦ�Ļ�ѧ����ʽΪ��Zn��2H2SO4(Ũ)=ZnSO4��SO2����2H2O��
�Ʒ�Ӧ����������H2SO4�����ʵ���Ϊ![]() ���ʴ�Ϊ��1.8mol��
���ʴ�Ϊ��1.8mol��
������A�����ʵ���![]()
�������⣬п�����ᷴӦ�Ļ�ѧ����ʽ�ǣ�
Zn��2H2SO4(Ũ)��ZnSO4��SO2����2H2O��
Zn��H2SO4(ϡ)��ZnSO4��H2����
�����ɵ�����ȫ�Ƕ�������������H2SO4�����ʵ���Ϊn(H2SO4)��2��n(SO2)��2��1.5 mol��3.0 mol��1.8 mol�����Դ˼��費��������������ֻ���Ƕ�������������Ļ�����塣
��û�������ж�����������������ʵ����ֱ���x��y����������ɵ������������̣�
x��y��1.5 mol��2x��y��1.8 mol��
���������x��0.3 mol��y��1.2 mol��![]() ���ʴ�Ϊ�����������������1��4��
���ʴ�Ϊ�����������������1��4��
�ȸ���������ѧ����ʽ��֪n(Zn)��n(SO2)��n(H2)��1.5 mol����m(Zn)��65 g��mol��1��1.5 mol��97.5 g���ʴ�Ϊ��97.5g��