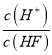��Ŀ����
����Ŀ���״���һ�ֿ������������Դ�����й����Ŀ�����Ӧ��ǰ����
��1����֪ ��CH3OH(g)+H2O(l)=CO2(g)+3H2(g) ��H= + 93.0kJ��mol��1
��CH3OH(g)+![]() O2(g)=CO2(g)+2H2(g) ��H=��192.9 kJ��mol��1
O2(g)=CO2(g)+2H2(g) ��H=��192.9 kJ��mol��1
�ۼ״���ȼ����Ϊ726.51kJ��mol-1��
Ҫд����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽ����ȱ�ٵ��Ȼ�ѧ����ʽΪ________________��
��2���״��ɲ���ú��������Һ����ȡ(CO+2H2CH3OH ��H<0)����T1��ʱ�����Ϊ2L�ĺ��������г������ʵ���֮��Ϊ3mol��H2��CO����Ӧ�ﵽƽ��ʱCH3OH���������(V%)��![]() �Ĺ�ϵ��ͼ��ʾ��
�Ĺ�ϵ��ͼ��ʾ��
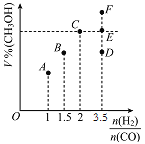
�ٵ���ʼ![]() =2������5min�ﵽƽ�⣬0~5min��ƽ����Ӧ����v(H2)=0.1molL-1min-1���������CO��ƽ��ת����Ϊ_____���������������䣬��T2��(T2>T1)�´ﵽƽ��ʱCO���������������____(����)
=2������5min�ﵽƽ�⣬0~5min��ƽ����Ӧ����v(H2)=0.1molL-1min-1���������CO��ƽ��ת����Ϊ_____���������������䣬��T2��(T2>T1)�´ﵽƽ��ʱCO���������������____(����)
A.<![]() B.=
B.=![]() C.
C.![]() ~
~ ![]() D.=
D.=![]() E.>
E.> ![]()
�ڵ�![]() =3.5ʱ���ﵽƽ��״̬��CH3OH���������������ͼ���е�____��ѡ����D������E������F��)��
=3.5ʱ���ﵽƽ��״̬��CH3OH���������������ͼ���е�____��ѡ����D������E������F��)��
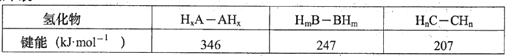
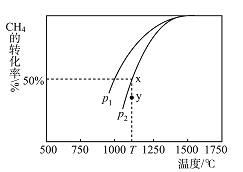
��3���Ƽ״���CO��H2������Ȼ������ȡ��CO2(g)+CH4(g)2CO(g)+2H2(g)����ijһ�ܱ���������Ũ�Ⱦ�Ϊ0.1mol��L1��CH4��CO2����һ�������·�Ӧ�����CH4��ƽ��ת�������¶ȼ�ѹǿ�Ĺ�ϵ��ͼ��ʾ����÷�Ӧ����H______(ѡ������������С����������������0��ѹǿp1_______(ѡ��������������С����)p2����ѹǿΪp2ʱ����y�㣺v(��)__________(ѡ������������С��������������)v(��)����p2=6Mpa����T��ʱ�÷�Ӧ��ƽ�ⳣ��Kp=_____MPa2����ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ=��ѹ�����ʵ�����������
��4���о�������CO2��H2��һ��������Ҳ���Ժϳɼ״�����Ӧ����ʽΪCO2(g)+3H2(g)CH3OH(g)+H2O(g) [��Ӧ��]��
��һ�������£���2L�����ܱ������г���2.0mol CO2��4.0mol H2���ڲ�ͬ���������ºϳɼ״�����ͬʱ����CO2��ת�������¶ȱ仯����ͼ��ʾ�����л����ߵķ�Ӧ���õĴ�����____(����A������B������C��)��

����ij���������£�CO2��H2��������Ӧ���⣬���������·�ӦCO2(g)+H2(g)CO(g)+H2O(g)[��Ӧ��]��ά��ѹǿ���䣬���̶���ʼͶ�ϱȽ�CO2��H2��һ������ͨ���ô�����������ͬʱ����ʵ�����ݣ�
T(K) | CO2ʵ��ת���ʣ�%�� | �״�ѡ���ԣ�%�� |
543 | 12.3 | 42.3 |
553 | 15.3 | 39.1 |
ע���״���ѡ������ָ������Ӧ��CO2��ת��Ϊ�״��İٷֱȡ�
��������˵���������¶ȣ�CO2��ʵ��ת������߶��״���ѡ���Խ��ͣ���ԭ����_________��
���𰸡�CH3OH(g)=CH3OH(l) ��H=38.19kJ/mol 50% C D �� �� �� 16 C �����¶ȣ���Ӧ�������ķ�Ӧ���ʾ��ӿ죬����Ӧ���ķ�Ӧ���ʱ仯����
��������
(1)�������⣬�״�ȼ���ȵ��Ȼ�ѧ����ʽ��CH3OH(l)+![]() O2(g)=CO2(g)+2H2O(l) ��H=726.51kJmol1�����ݸ�˹���ɼ��㣻
O2(g)=CO2(g)+2H2O(l) ��H=726.51kJmol1�����ݸ�˹���ɼ��㣻
(2)������������ƽ�ⷴӦ���ʣ�����������Ũ�ȱ仯������������COת���ʣ�CO+2H2CH3OH ��H��0��Ӧ���ȣ������¶ȶԻ�ѧƽ���ƶ���Ӱ�������
����![]() =2��1ʱ��ƽ��ʱ������ĺ������
=2��1ʱ��ƽ��ʱ������ĺ������![]() =3.5ʱ��CH3OH���������С�����ֵ��
=3.5ʱ��CH3OH���������С�����ֵ��
(3)����Ӱ�컯ѧƽ������ط������ɣ�����ͼ���������ʽ������ƽ�ⳣ����
(4)����T1�¶��£���ͬʱ���ڣ����Խ�ߣ���ӦԽ���ѣ�CO2ת������С��
���������ݱ����������¶ȣ����������¶ȣ���Ӧ�������ķ�Ӧ���ʶ��ӿ죬����Ӧ���ķ�Ӧ���ʱ仯����
(1)��֪ ��CH3OH(g)+H2O(l)=CO2(g)+3H2(g) ��H= + 93.0kJ��mol��1
��CH3OH(g)+![]() O2(g)=CO2(g)+2H2(g) ��H=��192.9 kJ��mol��1
O2(g)=CO2(g)+2H2(g) ��H=��192.9 kJ��mol��1
��CH3OH(l)+![]() O2(g)=CO2(g)+2H2O(l) ��H=726.51kJmol1��
O2(g)=CO2(g)+2H2O(l) ��H=726.51kJmol1��
���ݸ�˹���ɢ���3-����2-�ۿɵã�CH3OH(g)=CH3OH(l) ��H=38.19kJ/mol��
(2)��0��5min��ƽ����Ӧ����v(H2)=0.1molL-1min-1������c(H2)= v(H2)��t=0.1molL-1min-1��5min=0.5mol/L������CO+2H2CH3OH��CO��Ũ�ȱ仯��=0.25mol/L��H2��CO�ܹ�Ϊ3mol������ʼ![]() =2��1����֪H2Ϊ2mol��COΪ1mol��ƽ��ʱH2Ϊ1mol��COΪ0.5mol��CH3OHΪ0.5mol���������CO��ƽ��ת����=
=2��1����֪H2Ϊ2mol��COΪ1mol��ƽ��ʱH2Ϊ1mol��COΪ0.5mol��CH3OHΪ0.5mol���������CO��ƽ��ת����=![]() ��100%=50%����ͬ�����£����֮�ȵ������ʵ���֮�ȣ���ʱH2���������Ϊ
��100%=50%����ͬ�����£����֮�ȵ������ʵ���֮�ȣ���ʱH2���������Ϊ![]() =
=![]() ��CO+2H2CH3OH ��H��0��Ӧ���ȣ������������䣬��T2��(T2��T1)ʱ�������¶�ƽ�������ƶ��������ĺ������������������
��CO+2H2CH3OH ��H��0��Ӧ���ȣ������������䣬��T2��(T2��T1)ʱ�������¶�ƽ�������ƶ��������ĺ������������������
�ڻ�ϱ������ڻ�ѧ������֮��ʱ��ƽ��ʱ������ĺ�����ʵ�![]() =3.5ʱ���ﵽƽ��״̬��CH3OH���������С��C�㣬��ѡD��
=3.5ʱ���ﵽƽ��״̬��CH3OH���������С��C�㣬��ѡD��
(3)��ͼ���֪�������¶�CO��ת�������������¶ȣ�ƽ�����������ƶ������Է�ӦΪ���ȷ�Ӧ������H��0����ͼ��ɵã�����ͬ�¶��£�plʱCO��ת���ʴ���p2ʱCO��ת���ʣ��÷�Ӧ������Ӧ����Ϊ�����������ķ���ѹǿԽ��CO��ת��ԽС����pl��p2����ѹǿΪp2ʱ��y��CO��ת����С��ƽ��x���CO��ת���ʣ���y�㷴Ӧ������У�������y�㣬v(��)��v(��)������ͼ��ѹǿΪp2ʱ�������ת����Ϊ50%����������ʽ����

��p2=6Mpa��ƽ��ʱ�������ʵ������ʵ���Ϊ0.05+0.05+0.1+0.1=0.3mol����T��ʱ�÷�Ӧ��ƽ�ⳣ��Kp= =16MPa2��
=16MPa2��
(4)����ͼ��ʾ����T1�¶��£���ͬʱ���ڣ�����A�����µ�ת���������C�������µ�ת������С�����Խ�ߣ���ӦԽ���ѣ�CO2ת����Խ�ͣ��ʴ�ѡC��
�ڱ������ݱ��������������¶ȣ���Ӧ�������ķ�Ӧ���ʶ��ӿ죬����Ӧ���ķ�Ӧ���ʱ仯������ˣ��״���ѡ���Խ��ͣ���ԭ���������¶ȣ���Ӧ�������ķ�Ӧ���ʾ��ӿ죬����Ӧ���ķ�Ӧ���ʱ仯����