��Ŀ����
����Ŀ��ij��ɫ��Һ�п��ܺ���Na+��Ba2+��Cu2+��CO32����Cl����Mg2+�е�һ�ֻ������ӡ�
�������Һ�еμ�����ϡ��������������
��ȡ����������Һ������������Na2SO4��Һ���а�ɫ����������
��ȡ�����ϲ���Һ������������NaOH��Һ���а�ɫ����������
��1��ԭ��Һ��һ�����е�������________��һ�������е�������________������ȷ���Ƿ��е�������________��
��2�����з�Ӧ�����ӷ���ʽΪ___________________��
��3������ȡ10mL������Һ����ƿ�У�Ȼ�������Һ����μ���NaOH��Һ(��ͼ����ʾ)���μӹ����в������������������NaOH��Һ������Ĺ�ϵ��ͼ����ʾ��
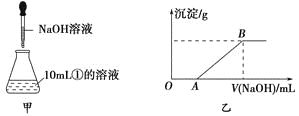
��OA�η�Ӧ�����ӷ���ʽΪ_____________________��
��AB�η�Ӧ�����ӷ���ʽΪ_______________________��
���𰸡�Ba2+��Cl����Mg2+ CO32����Cu2+ Na+ Ba2+��SO42��=BaSO4�� H����OH��=H2O Mg2����2OH��=Mg(OH)2��
��������
��ɫ��Һ��һ������Cu2+������Һ�еμ�����ϡ��������������һ��û��CO32����ȡ����������Һ������������Na2SO4��Һ���а�ɫ����������һ����Ba2+��ȡ�����ϲ���Һ������������NaOH��Һ���а�ɫ����������һ����Mg2+�����ݵ���غ㣬һ����Cl����
��1��ԭ��Һ��һ�����е�������Ba2+��Cl����Mg2+��һ�������е�������CO32����Cu2+������ȷ���Ƿ��е�������Na+��
��2�����з�Ӧ�����ӷ���ʽΪBa2+��SO42��=BaSO4����
��3��������Һ�к���Ba2+��Cl����Mg2+��H+���μ��������ƣ��������Ⱥ����������ӷ�Ӧ��Ȼ����Mg2+�����������ӷ�Ӧ������OA��ΪH����OH��=H2O��AB�η�Ӧ�����ӷ���ʽΪMg2����2OH��=Mg(OH)2����

 ���㼤�������100�ִ��Ծ�ϵ�д�
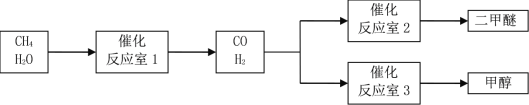
���㼤�������100�ִ��Ծ�ϵ�д�����Ŀ����CH4�� H2OΪԭ���Ʊ������Ѻͼ״��Ĺ�ҵ�������£�
��֪��2CO(g)��O2(g)��2CO2(g)��H����566 kJ��mol-1��CH3OCH3 (g)��3O2(g)��2CO2(g)��3H2O (g) ��H����1323 kJ��mol-1��2H2(g)��O2(g)��2H2O(g)��H����484 kJ��mol-1��
��1����Ӧ��3�з�����Ӧ��CO(g)��2H2(g)��CH3OH(g)���÷�Ӧ��һ�����������Է����е�ԭ����_____��
��2����Ӧ��2�з�����Ӧ��2CO(g)��4H2(g)��CH3OCH3(g)��H2O (g) ��H��_____��
��3����Ӧ��1�з�����Ӧ��CH4(g)��H2O (g) ![]() CO(g)��3H2(g)���Դ˷�Ӧ���������о���T��ʱ�����ݻ�Ϊ2 L���ܱ������г���һ������CH4(g)��H2O (g)���з�Ӧ��ʵ���÷�Ӧ�����еIJ������ݼ��±�������t1��t2����
CO(g)��3H2(g)���Դ˷�Ӧ���������о���T��ʱ�����ݻ�Ϊ2 L���ܱ������г���һ������CH4(g)��H2O (g)���з�Ӧ��ʵ���÷�Ӧ�����еIJ������ݼ��±�������t1��t2����
��Ӧʱ��/min | n(CH4)/mol | n(H2O)/ mol |
0 | 1.20 | 0.60 |
t1 | 0.80 | |
t2 | 0.20 |
�ٷ�Ӧ�ӿ�ʼ��t1����ʱ��ƽ����Ӧ����Ϊv(H2)=_______mol��L-1��min-1��
�������������������䣬��ʼʱ�������г���0.60 mol CH4��1.20 mol H2O����Ӧһ��ʱ����������H2�����ʵ���Ϊ0.60 mol�����ʱv��______v�����>������<����=������
����������Ӧ�ı�ijһ���������H2�����ʵ�����ʱ��仯��ͼ������B��AΪԭ��Ӧ�����ߣ�����ı������������_________��
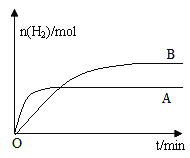
��4���Է�Ӧ��1������CO��H2Ϊȼ�ϣ�һ������Li2CO3��Na2CO3���ۻ����Ϊ����ʹ��ɵ�һ��̼����ȼ�ϵ��������ͼ��ʾ��
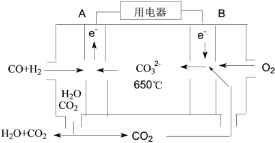
�ٸõ�ص�������ӦʽΪ_____��
������·������4 mol���ӣ�������������CO��H2�������Ϊ________L����״������