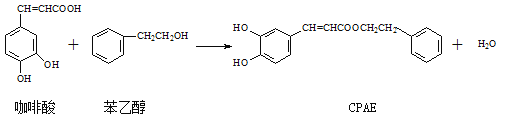
��Ŀ����
����Ŀ�������ͷǽ������������������еõ��˹㷺Ӧ��,����ش��������⡣
��1��������ͷ۵���Ҫ�ɷ�֮һ��һ�����Σ����׳�Ϊ________��Ư�۵���Ч�ɷ�Ϊ__________��д��ѧʽ����FeCl3��������ˮ������ԭ��Ϊ_________�������ӷ���ʽ��ʾ����
��2��մ��ˮ�����������ڸ��»����ϻᷢ�ڣ��÷�Ӧ�Ļ�ѧ����ʽ��_________��
��3������Ѫ�쵰���к���Fe2+������ʳ�������Σ��ᵼ��Fe2+ת��Fe3+���ж�������ά����C���Խⶾ���������ķ�����ȷ������______��
A�����������ǻ�ԭ�� B��ά����C��������
C��ά����C������ D���������η���������Ӧ
��4��ijCuSO4��H2SO4�Ļ����Һ100mL����֪��Һ�������ӵ�Ũ����ȣ�������ˮ�⣩����SO42-�����ʵ���Ũ��Ϊ6mol/L�������Һ�������ӵ�Ũ��Ϊ___________mol/L���������Һ�м������Zn��������ܽ�Zn������Ϊ____________g��
���𰸡�С�մ� Ca(ClO)2 Fe3++ 3H2O ![]() Fe(OH)3(����)+3H+ 3Fe��4H2O(g)
Fe(OH)3(����)+3H+ 3Fe��4H2O(g) ![]() Fe3O4��4H2 C 4 39.0
Fe3O4��4H2 C 4 39.0
��������
��1��������ͷ۵���Ҫ�ɷ�֮һ̼�����ƣ����׳�ΪС�մ�ҵ����������ʯ���鷴Ӧ���ɴ�����ơ��Ȼ��ƺ�ˮ�������Ȼ��ƺʹ��������Ư�۵���Ҫ�ɷ֣�Ư�۵���Ч�ɷ�Ϊ������ƣ���ѧʽΪCa(ClO)2��FeCl3����ǿ�������Σ����������ӷ���ˮ�⣬���������������壬������м������ԣ�������ˮ�еĹ������ʣ���������ˮ�������ӷ���ʽΪFe3++ 3H2O ![]() Fe(OH)3(����)+3H+��
Fe(OH)3(����)+3H+��
��Ϊ��С�մ�Ca(ClO)2��Fe3++ 3H2O ![]() Fe(OH)3(����)+3H+��
Fe(OH)3(����)+3H+��
��2��������������ˮ����������Ӧ�����������������������÷�Ӧ�Ļ�ѧ����ʽ��3Fe��4H2O(g) ![]() Fe3O4��4H2��
Fe3O4��4H2��
����3Fe��4H2O(g) ![]() Fe3O4��4H2��
Fe3O4��4H2��
��3������Ѫ�쵰���к���Fe2+������ʳ�������Σ��ᵼ��Fe2+ת��Fe3+���ж������Ļ��ϼ����߱�������˵���������ξ��������ԣ���������������ά����C���Խⶾ��������ʹFe3+ת��Fe2+�����ά����C���л�ԭ�ԣ�����ԭ����
A����������������������A����
B��ά����C�ǻ�ԭ������B����
C��ά����C�ǻ�ԭ������Ӧ�б���������C��ȷ��
D����������������������Ӧ�з�����ԭ��Ӧ����D����
��ѡC��
��4��CuSO4��H2SO4�Ļ����Һ100mL������������Ϊͭ���Ӻ������ӣ�������Ϊ��������ӣ���֪��Һ�������ӵ�Ũ����ȣ�����Һ�������ӵ�Ũ��Ϊx mol/L��������Һ�е���غ�ɵ�xmol/L��2+xmol/L��1=6mol/L��2����ã�x=4 mol/L��������Һ�м���п������ͭ�����ᶼ������п��Ӧ��п��ͭ���ӷ�Ӧ��Zn+Cu2+�TZn2++Cu�����ܽ�п�����ʵ���=ͭ���ӵ����ʵ���=4 mol/L��0.1L=0.4mol��п�������ӷ�Ӧ��Zn +2H+�TZn 2++H2�������ܽ�п�����ʵ���=�����ӵ����ʵ���һ��=4 mol/L��0.1L��![]() =0.2mol�����ϣ��û����Һһ���ܽ�п�����ʵ���Ϊ0.2 mol +0.4 mol =0.6mol�����ܽ�п������=0.6mol��65g/mol=39g
=0.2mol�����ϣ��û����Һһ���ܽ�п�����ʵ���Ϊ0.2 mol +0.4 mol =0.6mol�����ܽ�п������=0.6mol��65g/mol=39g
����4��39��