��Ŀ����
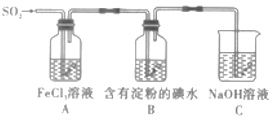
����Ŀ���ҹ���ѧ�Һ�°����NaHCO3�ܽ�ȱ�NaCl��Na2CO3��NH4HCO3��NH4Cl��С�����ʣ�����CO2 + NH3 + H2O + NaCl = NaHCO3��+ NH4Cl�ķ�Ӧԭ���Ʊ������������ʵ���ҽ���ģ��ʵ�����������ʾ��ͼ��

�����������������( )
A.A������NH3��B������CO2
B.�ڢõ��ľ����Ƿ��ͷ۵���Ҫ�ɷ�
C.�ڢ����õ�����Ҫ�����������ձ���©����������
D.�ڢ�����������Ҫ�������ܽ⡢�������ᾧ
���𰸡�D
��������
A�����ڰ�����ˮ�е��ܽ�ȴ�����Ӧ����ˮ�м���������NaCl�γɱ�����Һ�������ڼ��Ի����п�������CO2�ܽ�ȣ���ͨ��CO2���壬�Ϳ�������CO2���ܽ�ȣ��γɸ����NaHCO3������Һ�����Ժ���γɳ���������������ˮ������ͨ��CO2�������������Ի����ܽ��С����Һ���γɵ�NaHCO3�٣��������ľ���Ҳ�٣�����A������NH3��B������CO2��ѡ��A��ȷ��
B���ڢõ��ľ�����NaHCO3���Ƿ��ͷ۵���Ҫ�ɷ֣�B��ȷ��
C���ڢ��˲��������õ�����Ҫ�����������ձ���©������������C��ȷ��
D���ڢ�����̼���������ȷֽ⣬��������Ҫ������ϴ�ӡ����ȣ�D����
��ѡD��

 ����������������ϵ�д�
����������������ϵ�д�����Ŀ��ijʵ��С��������ͼ��ʾװ����ʵ�������Ʊ�����������
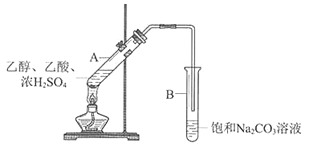
��1��Bװ���У����ܿ��Ը���Һ���Ŀ����________________________
��2��Ϊ�˸��Ʒ�Ӧ�����л����Һ��ڵ�����ͬѧ����NaHSO4����Ũ�����������������Լ����������䣩���Ʊ�����������NaHSO4�ɴ���Ũ������������ԭ����__________________________________________________________
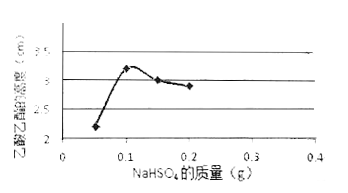
��3����ͼ�Ǽ�ͬѧ����NaHSO4�������������������䣨������������Ϊ2 mL����ͬ������NaHSO4���Ʊ����������Ĵ�Ч��ͼ������NaHSO4������Ϊ_______gʱ��Ч����á�
��4����ͬѧʵ��ʱ,�ڱ���NaCO3�еμ����η�̪��ʵ�������ȡ���Թ�B����ɫ��ȥ��Ϊ̽����ɫ��ԭ��������ʵ�顣
��� | �� | �� | �� |
ʵ�� |
|
|
|
���� | ��������� | ��������� | ��������á���Һ��ȡ�²���Һ�����뱥��Na2CO3��Һ |
���� | �ϲ�Һ��䱡��ð���ݣ��²���Һ��ɫ��ȥ | �ϲ�Һ�岻�䱡�������ݣ��²���Һ��ɫ��ȥ |
a�Թܢ��в������ݵ�ԭ���ǣ��û�ѧ����ʽ���ͣ�____________________________��
b�Ա�ʵ��ٺ͢ڿɵó��Ľ�����___________________________________��
c���ʵ�����������ͬѧ������룺��̪������������������ʵ����й۲쵽__________________________________��֤ʵ�ҵIJ�����ȷ��