��Ŀ����
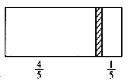
����Ŀ��һ���ܱ��������м���һ�����ɻ����ĸ���(��ȿɺ���)�������ֳ������֣���������1 mol N2���Ҳ����CO��CO2�Ļ�����干8 gʱ�����崦����ͼλ��(���������¶���ͬ)������˵����ȷ����
A. �Ҳ�CO��CO2������֮��Ϊ1��3
B. �Ҳ������ܶ�����ͬ�����������ܶȵ�18��
C. �Ҳ�CO������Ϊ1.75 g
D. �����崦�ھ����Ҷ�1/6���������������䣬��ǰ������ѹǿ֮��Ϊ25��24
���𰸡�D
��������
�����߳���1molN2���ұ߳���CO��CO2�Ļ�����干8g�������ҵ�ѹǿ��ȣ���������CO��CO2�������ʵ���Ϊ1mol��![]() =0.25mol����CO��CO2�����ʵ����ֱ�Ϊxmol��ymol����
=0.25mol����CO��CO2�����ʵ����ֱ�Ϊxmol��ymol����![]() �����x=0.1875��y=0.0625��
�����x=0.1875��y=0.0625��
A��������Ŀ֮�ȵ������ʵ���֮�ȣ�
B������![]() =
=![]() ����ƽ��Ħ����������ͬ�����������ܶ�֮�ȵ�����Ħ������֮�ȣ�
����ƽ��Ħ����������ͬ�����������ܶ�֮�ȵ�����Ħ������֮�ȣ�
C������m=nM����CO��������
D�������崦�ھ����Ҷ�![]() �����������������ʵ���Ϊ1mol��
�����������������ʵ���Ϊ1mol��![]() =0.2mol����PV=nRT�ɵ�P=cRT�������������ѹǿ֮�ȵ���������Ũ��֮�ȣ�
=0.2mol����PV=nRT�ɵ�P=cRT�������������ѹǿ֮�ȵ���������Ũ��֮�ȣ�
�⣺����߳���1molN2���ұ߳���CO��CO2�Ļ�����干8g�������ҵ�ѹǿ��ȣ���������CO��CO2�������ʵ���Ϊ1mol��![]() =0.25mol����CO��CO2�����ʵ����ֱ�Ϊxmol��ymol����
=0.25mol����CO��CO2�����ʵ����ֱ�Ϊxmol��ymol����![]() �����x=0.1875��y=0.0625��
�����x=0.1875��y=0.0625��
A��������Ŀ֮�ȵ������ʵ���֮�ȣ��ұ�CO��CO2������֮��Ϊ0.187mol��0.0625mol=3��1����A����
B���ɴ������ƽ��Ħ������Ϊ![]() =32g/mol����ͬ�����������ܶ�֮�ȵ�����Ħ������֮�ȣ����Ҳ������ܶ�����ͬ�����������ܶȵ�
=32g/mol����ͬ�����������ܶ�֮�ȵ�����Ħ������֮�ȣ����Ҳ������ܶ�����ͬ�����������ܶȵ�![]() =16������B����
=16������B����
C��CO������Ϊ0.1875mol��28g/mol=5.25g����C����
D����PV=nRT�ɵ�P=cRT�������������ѹǿ֮�ȵ���������Ũ��֮�ȣ������崦�ھ����Ҷ�![]() �����������������ʵ���Ϊ1mol��
�����������������ʵ���Ϊ1mol��![]() =0.2mol����ǰ������ѹǿ֮��Ϊ
=0.2mol����ǰ������ѹǿ֮��Ϊ![]() ��
��![]() =25��24����D��ȷ��
=25��24����D��ȷ��
��ѡ��D��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�